The major difference between a primary cell and the secondary cell is that primary cells are the ones that cannot be charged but secondary cells are the ones that are rechargeable. Before heading towards the discussion of differences between both cells, let us learn about them in brief.
Interesting Science Videos
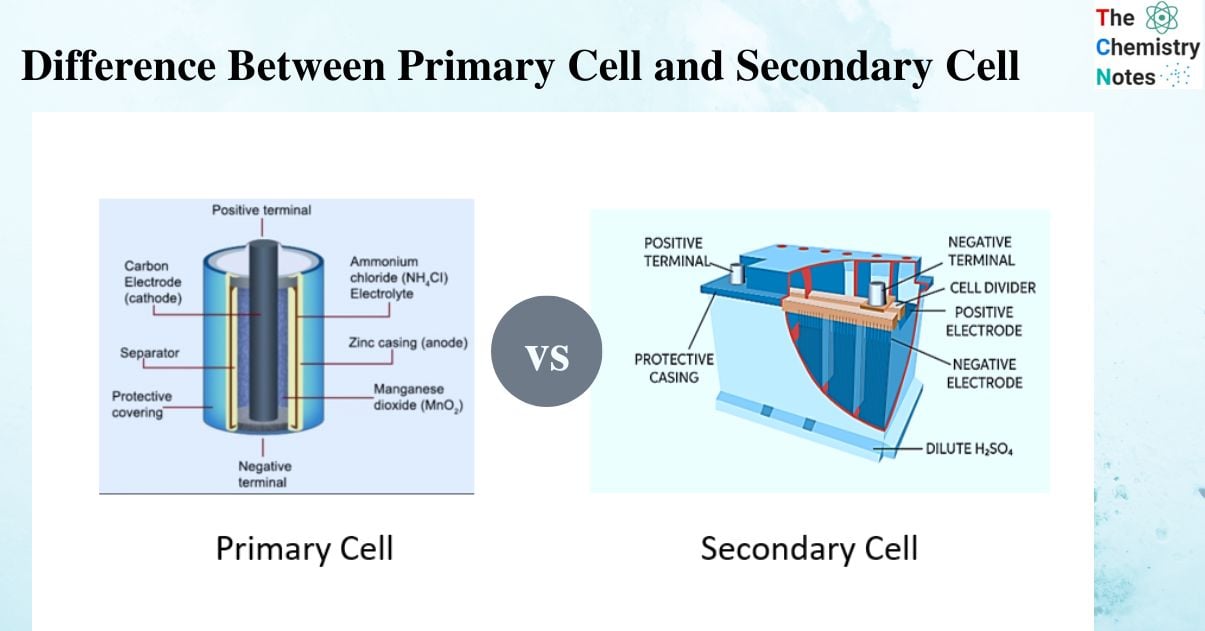
What is Primary Cell?
Non-rechargeable batteries are also known as primary cells or primary batteries. This is primarily due to the fact that once these batteries are depleted, they cannot be recharged. Although they are not reusable, primary cells are very useful for storing power for long-term use due to their low self-discharge rate. As a result, they are used in service pacemakers for heart patients, smart meters, and military campaigns where charging is impractical or impossible.
Because of the irreversible chemical reactions that occur inside the battery, primary cells are non-rechargeable. The chemical reactions use all of the chemicals in the cell, and once all of the chemical species are used, power generation stops.
A primary battery or primary cell is made up of an anode (positively charged end) and a cathode (negatively charged end) (negatively charged end). The cathode is typically graphite, and the anode is zinc. The anode undergoes oxidation reactions in which electrons are donated to the circuit, while the cathode undergoes reduction reactions in which electrons are accepted from the outside. There is also an electrolyte, which aids in the passage of electric current. The electrolyte is made up of electrically charged ions that can transfer their charge between the cathode and the anode.
A common example of primary cell is Leclanche cell. It is composed of a Zinc anode and porous graphite cathode. The electrolyte present inside the battery is a moist mixture of NH4Cl (ammonium chloride), Zinc chloride (ZnCl2) and Manganese dioxide (MnO2).
Chemical Reaction
The chemical reactions that occur inside the cell can be given as below.
Anode: Zn (s) → Zn2+(aq) + 2e
Cathode: 2NH4+(aq) + 2e → 2NH3 (g) + H2 (g)
Hence two gases NH3 and H2 are produced in the cathode. But these gases again will participate in reactions as shown below.
2NH3 (g) + Zn2+(aq) → [Zn(NH3)2]2+(aq)
2MnO2 (s) + H2 (g) →Mn2O3 (s) +H2O (l)
What is a Secondary Cell?
Secondary cells are also known as rechargeable batteries or secondary batteries. Because they can be recharged, these batteries can be used multiple times. Reversible chemical reactions occur in these batteries. Thus by applying an electrical charge, those reactions can be reversed. Secondary batteries, unlike primary batteries, must be charged before use. Chargers are used to recharge batteries.
Different secondary batteries however serve with different purposes. As a result, the user should understand which type of battery to use for a specific need.
These batteries, like primary batteries, also have a cathode and anode. Reduction reactions take place in the cathode, whereas oxidation reactions take place in the anode. The lead storage/acid battery is hence an excellent example of secondary cells.
Chemical Reaction
The reactions that occur within that cell are depicted below.
Cathode: PbO2 (s) + HSO4–(aq) + 3H+(aq) + 2e → PbSO4 (s) + H2O (l)
Anode: Pb (s) + HSO4–(aq) → PbSO4 (s) + H+(aq) + 2e
Difference Between Primary Cell and Secondary cell
| Primary Cell | Secondary cell | |
| Definition | Primary cells are batteries that cannot be easily recharged after use. | Secondary cells are batteries that can be recharged and reused. |
| Chemical Reactions | In primary cells, irreversible reactions occur. | In primary cells, reversible reactions occur. |
| Design | Primary cells are frequently referred to as “dry cells” due to the technology used to create them. Because there are no fluids in the battery, but the cells are filled with paste that allows the ions to move but prevents them from spilling. | Secondary cells make use of the other two types of cells: wet cells (flooded cells) and molten salt cells (liquid cells with a slightly different composition). |
| Usage | Primary cells can be used only once. | Secondary cells can be used more than once. |
| Specifications | Primary cells have a high internal resistance, an irreversible chemical reaction, a higher capacity, are typically smaller and lighter, and are generally less expensive. | Secondary cells have a lower internal resistance, require charging, have reversible chemical reactions, and are more complex and costly. |
| Discharge | In comparison, it has a slow discharge. | Comparatively higher discharge. |
| Application | Primary cells are used in devices that require a small but constant current, such as clocks, toys, and safety equipment. | Secondary cells are used in portable devices – laptops, mobile phones, mp3 players, tablets, etc. |
| Cost | Primary cells are cheap and easy to use and these cell are cost effective compared to secondary cells, initially. | Secondary cells are expensive and more complex in usage. however, using these cells would be a long term investment since primary cells are to be replaced by another set after some time. |
References
- https://pediaa.com/difference-between-primary-and-secondary-cells/
- https://byjus.com/chemistry/difference-between-primary-cell-and-secondary-cell/#:~:text=The%20major%20difference%20between%20a,the%20ones%20that%20are%20rechargeable.
- https://www.electricalvolt.com/2022/02/difference-between-primary-cell-and-secondary-cell/
- https://askanydifference.com/difference-between-primary-cell-and-secondary-cell/
- https://www.batterypowertips.com/difference-between-primary-secondary-battery- chemistries- faq/
- https://www.differencebetween.com/difference-between-primary-and -vs-secondary- cells/
- https://unacademy.com/content/nda/study-material/chemistry/ electrochemistry-battery_ chemistry/
