Adsorption
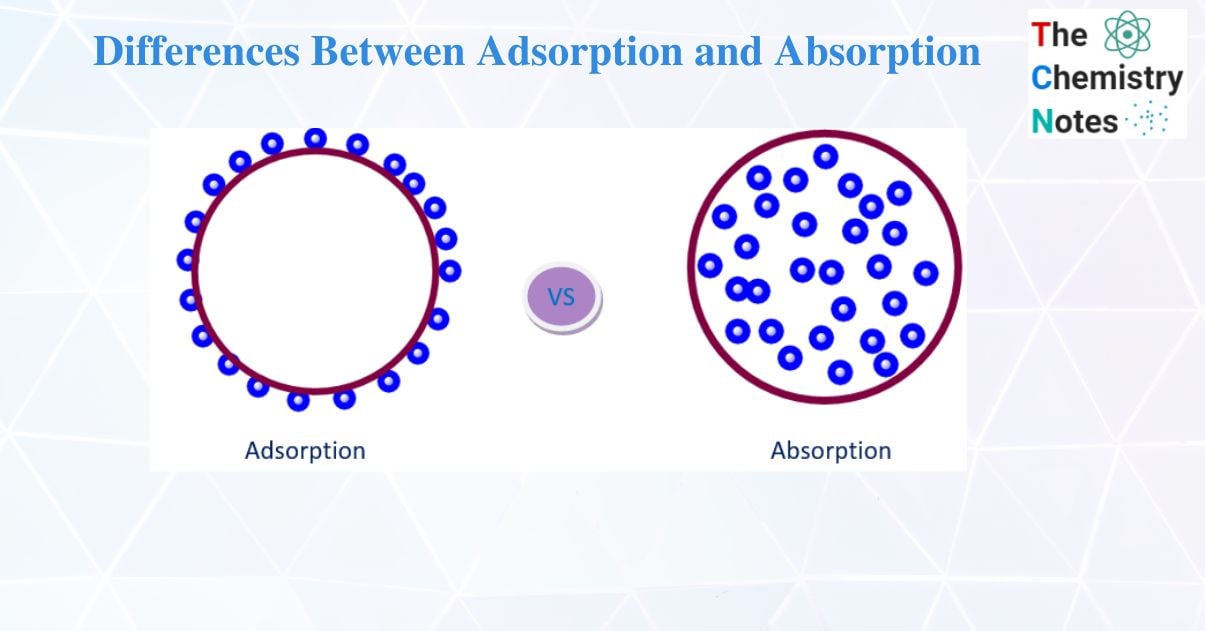
Adsorption is a surface phenomenon that involves the accumulation of molecular species on the surface rather than within the bulk of a solid or liquid. Adsorbate is the substance that accumulates on the surface of another substance. For instance, H2, N2, and O2 gases. The adsorbent is the material on the surface on which adsorption occurs. For example, Charcoal, Silica gel.
Adsorption can be classified into two types. They are:
- Chemisorption: It is an irreversible phenomenon that involves the strong chemical interaction between adsorbate and adsorbent. For example, Iron on heating with N2 gas at 623 K produces iron nitride on the surface.
- Physisorption: The reversible phenomenon involves the weak Van der Waals forces between adsorbate and adsorbent. E.g., the Adsorption of H2 on the surface of the charcoal.
Absorption
It involves the assimilation of the molecular system across the entire solid or liquid medium. Absorption can be classified into two types. They are:
Chemical absorption: Chemical absorption is reactive absorption that involves the reaction between the absorbed and absorbing substances.
Physical absorption: Physical absorption is a non-reactive process, during which the electronic structure of the atom or molecule do not perturbe upon adsorption.
Differences between adsorption and absorption
| S.N., | Adsorption | Absorption |
| 1. | Adsorption is a surface phenomenon that involves the accumulation of molecular species on the surface rather than within the bulk of a solid or liquid | Assimilation of the molecular system across the entire solid or liquid medium. |
| 2. | The substance is concentrated only at the surface and does not penetrate through the surface to the bulk of the adsorbent | The substance is uniformly distributed throughout the bulk of the solid |
| 3. | It is a surface phenomenon. Molecules that are undergoing the adsorption process are taken by the surface. | It is a bulk phenomenon. Molecules that are undergoing the absorption process are taken within the molecules. |
| 4. | It is an exothermic process. | It is an edothermic process. |
| 5. | Low temperature favors the adsorption process. | Absorption is not affected by temperature. |
| 6. | The concentration of adsorbent on the surface differs from that in the bulk. | Concentration is the same throughout the material. |
| 7. | It is used in water purification, vegetable and animal oil refining, hydrocarbon fractionation, and other applications. | It is used in Cold storage, ice production, cooling, and refrigerants. |
References
- Hiemenz P. C. & Rajagopalan R. (1997). Principles of colloid and surface chemistry (3rd ed. rev. and expanded Paul C. Hiemenz Raj Rajagopalan). Marcel Dekker.
- https://sciencenotes.org/adsorption-vs-absorption-differences-and-examples/
- https://nitsri.ac.in/Department/Chemical%20Engineering/Adsorption.pdf
- https://www.britannica.com/science/absorption-physics
- https://www.che.ncku.edu.tw/facultyweb/leeyl/94%20%E7%95%8C%E9%9D%A2%E7%8F%BE%E8%B1%A1/Chapter%2009%20Adsorption.pdf
