There are two different kinds of electrochemical cells: galvanic cells, which have spontaneous redox reactions that permit continuous electron flow through the conductor, converting chemical energy into electrical energy; and electrolytic cells, which have redox reactions that are influenced by an external source of current, converting electrical energy into chemical energy.
What is Galvanic Cell?
Galvanic cells are devices that convert chemical energy into electrical energy, which produces current.
The redox (oxidation-reduction) process in galvanic cells results in the production of direct current. Two halves of a cell make up the galvanic element. The electrolyte and the immersed electrode make up the half-cell. A contact must be established between these half-cells, connecting the electrode to a conductor and the electrolyte to a salt bridge or semiconductive membrane. The behavior of the electrodes in relation to the electrolyte explains how the sepration of redox process takes place.
The most straightforward option is to have a metal electrode submerged in an electrolyte that contains ions that correspond to the electrode. The reactivity of the metal, or its propensity to dissolve, determines how it behaves in the electrolyte.
What is an Electrolytic Cell?
An electrolytic cell is a type of electrochemical cell that employs external electrical energy to fuel a chemical reaction.
Two electrodes (anode and cathode) and an electrolyte make up the three main components of a typical electrolytic cell. where the ions are dissolved in a water-molten slat solution, such as sodium chloride, which serves as the electrolyte. It is possible to spontaneously induce electrode reactions on both phases of the metal/electrolyte by cutting the electric circuit. Additionally, the energy of a spontaneous chemical reaction is sacrificed in order to release the energy of the current. Another method of closing an electrical circuit involves serially bonding a current source from outside the cell with a voltage source from outside the cell, where the external voltage is higher than the electromotive force of the cell. It causes the current to flow through the cell in the opposite direction from the way it naturally does.
As a result, the cell’s reactions involving electrodes must be at odds with the direction of their spontaneous flow. An electrochemical cell in this mode of operation is an electrolytic cell. Electrolysis is the term for forced processes in an electrochemical cell under the influence of an external source of electrical current.
Difference between Galvanic vs Electrolytic Cell
The main distinction is that galvanic cells convert chemical energy into electrical energy, whereas electrolytic cells convert electrical energy into chemical energy. As well as the positive and negative charges are in different positions in the cells.
Galvanic vs Electrolytic Cell
| Galvanic Cell | Electrolytic Cell | |
| Definition | A galvanic cell is an electrochemical cell that uses a chemical reaction to generate electricity. | An electrolytic cell is a type of cell that advances a chemical reaction using an electric current. |
| Technique | A galvanic cell transforms chemical energy into electrical energy. | Electrical energy is transformed into chemical energy by an electrolytic cell. |
| Chemical Reaction | A spontaneous reaction occurs. | A non-spontaneous reaction occurs. |
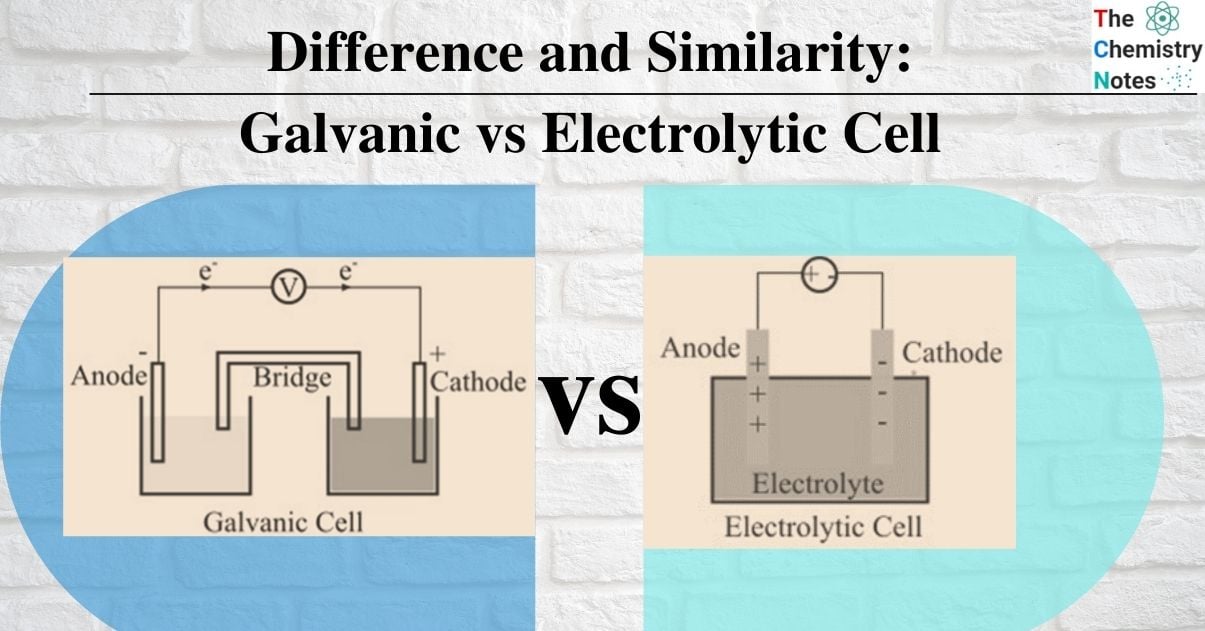
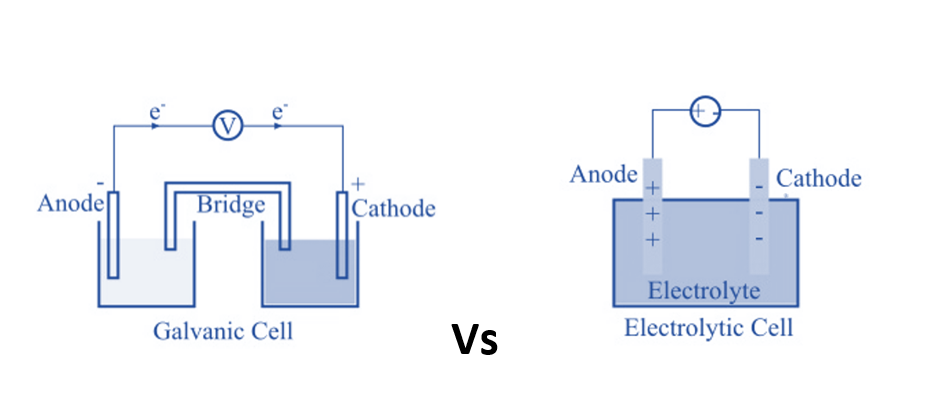
| Anode and Cathode | The anode is negatively charged, and the cathode is positively charged. | The anode is positively charged, and the cathode is negatively charged. |
| External Source | Does not require external voltage source | Require external voltage source. |
| Design | A semipermeable membrane or a salt bridge separates two different electrodes that are submerged in solutions of their ions in a galvanic cell. | An electrolytic container with two electrodes connected to a DC source makes up an electrolytic cell. The electrolyte may be a melt or an aqueous solution of some salt, acid or alkali. |
| Oxidation and Reduction | Oxidation takes place at the anode and reduction at the cathode. | Oxidation takes place at the cathode and reduction at the anode |
| Flow of electrons | Electrons being negatively charged flow from anode to cathode of the galvanic cell. | Electrons flow from the cathode to the anode in an electrolytic cell. |
| Number of cell containers | Two separate cell containers that form two half cells connected by a salt bridge. | A single cell container forms the complete electrolytic cell. |
| Ions on electrodes | Ions discharge at the cathode and consume at the anode. | Both the electrodes discharge the ions. |
| Application | Also known as batteries or accumulators, are used as a source of electrical current. | Production of hydrogen and oxygen gas for use in commerce and industry, electroplating, removing pure metals from alloys, and other uses. |
Similarities Between Galvanic vs Electrolytic Cell
Type of Cell: Both are electrochemical cells
Anode and cathode reactions
Oxidation happens at the anode and reduction happens at the cathode in voltaic and electrolytic cells, respectively. Thus, redox reactions are present in both of these cells.
Electron Flow Direction
In both of these cells, electrons move through the externally connected conductor from the anode to the cathode.
Read more: Differences Between Primary Cell and Secondary Cell
References
- https://avesis.marmara.edu.tr/yayin/17177586-0673-4859-9b7c-66eafdae92d7/prospective-teachers-conceptual-understanding-of-electrochemistry-galvanic-and-electrolytic-cells
- https://sciencestruck.com/similarities-differences-between-voltaic-electrolytic-cells
- https://byjus.com/chemistry/difference-between-galvanic-cells-and-electrolytic-cells/
- https://askanydifference.com/difference-between-galvanic-cells-and-electrolytic-cells-with-table/
