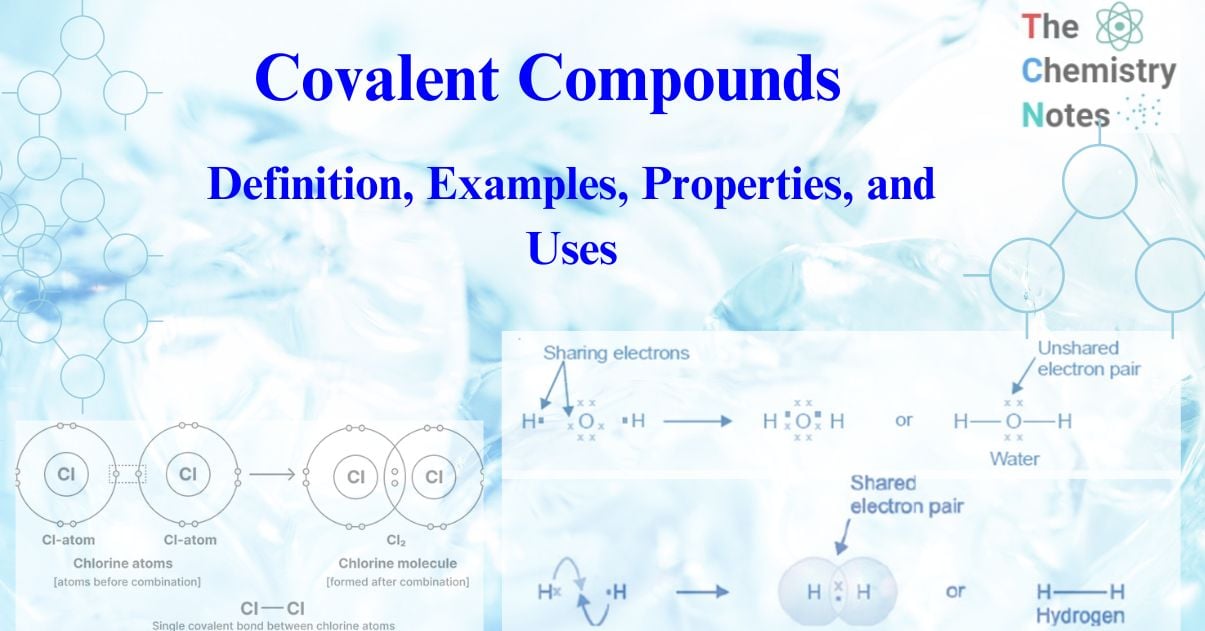
The compounds containing a covalent bond are called covalent compounds. The formation of a covalent bond occurs as a result of a decrease in the overall energy of the bonded atoms relative to that of the atoms that are widely separated. In general terms, the formation of a covalent bond is achieved through an equal sharing of electrons between the two atoms involved in the bond. The electrons involved in this form of bonding are referred to as a shared pair or bonding pair. The term “covalent bonds” is often used interchangeably with “molecular bonds.” The act of sharing bonding pairs between atoms facilitates the attainment of outer shell stability, a characteristic shared by noble gases.
What is a covalent bond?
Elements with high ionization energies are unable to undergo electron transfer, while elements with low electron affinity are unable to accept electrons. Elements of this nature exhibit a tendency to participate in electron sharing with atoms of either the same or different elements. This process enables both participating atoms to attain an octet configuration within their respective valence shells, thereby achieving a state of stability. The phenomenon of sharing electron pairs between similar or dissimilar entities is referred to as a Covalent Bond.
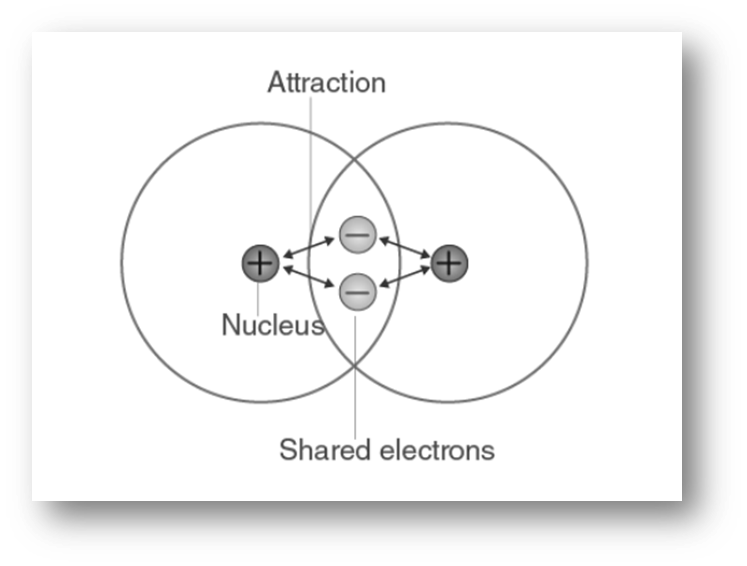
Conditions for the formation of covalent bond
- The number of valence electrons: For the attainment of a stable octet through the sharing of electron pairs, it is necessary for both atoms A and B to possess 5, 6, or 7 valence electrons. The element H possesses a single electron in its valence shell and achieves a duplet configuration. The elements belonging to groups VA, VIA, and VIIA exhibit this characteristic and are classified as non-metals.
- Equal electronegativity: When the electronegativity of atom A is equivalent to that of atom B, electron transfer between them is unlikely to occur. Instead, electron sharing is expected to take place. This scenario is only feasible under the condition that both atoms are of identical chemical composition.
- Equal sharing of electrons: In order, for atoms A and B to attract the bonding electron pair in an equivalent manner, it is necessary for their electron affinities to be comparable, or nearly comparable. A nonpolar covalent bond will be formed as a result of the equitable sharing of electrons. Because no two elements have exactly the same electron affinity, absolutely equal sharing of electrons will not normally occur until when atoms A and B are both of the same element. This is the only circumstance in which exactly equal sharing of electrons will occur.
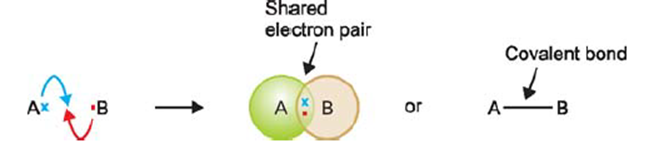
A compound consisting only of covalent bonds is referred to as a covalent compound. Typically, a chemical bond of this nature occurs between two non-metallic elements or between a non-metallic element and a metalloid, which exhibits characteristics of both metals and non-metals. Covalent compounds are characterized by possessing strong intra-molecular bonds. The reason for this phenomenon is attributed to the strong interatomic bonding within the covalent molecules. The discrete nature of each molecule is a characteristic feature of covalent compounds, and the intermolecular forces governing their behavior are typically of low magnitude.
The energy required for molecular separation is minimal. The aforementioned phenomenon can be attributed to the intermolecular forces that exist between the molecules, which are not accompanied by any apparent net electric charge. Typically, molecules of covalent compounds exist in a gaseous state under standard temperature and pressure conditions. These substances could also potentially exist in a liquid state and possess comparatively low boiling points. The mentioned features may be attributed to the relatively weak intermolecular forces that bind these atoms together.
Covalent compounds formed as a result of the similarity or identity of the electronegativity values of the constituent elements in the compound. Typically, atoms tend to participate in covalent bonding when the difference in electronegativity is below 2 on the Pauling scale. When the difference in electronegativity surpasses two, the chemical elements engage in the formation of ionic bonds. One illustrative example is carbon dioxide (CO2).
An exception to the previously mentioned principle is that if a molecule comprises only of nonmetallic elements, it is classified as a covalent compound. Nevertheless, there exists an interesting Exception. Due to its high electropositivity, the ammonium cation (NH4+) tends to engage in ionic bonding with nonmetals, as opposed to covalent bonding. Simultaneously, the nitrogen and hydrogen atoms exhibit covalent bonding. The chemical compounds of ammonium chloride (NH4Cl) and ammonium nitrate (NH4NO3) are characterized by the presence of both ionic and covalent bonds.
Examples of covalent compounds
Some of the examples of covalent compounds are discussed below:
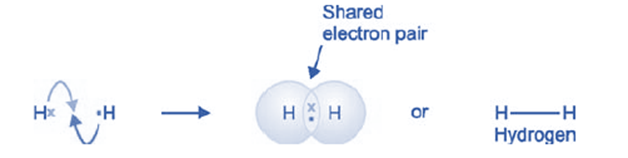
Hydrogen (H2)
The hydrogen molecule is composed of a diatomic pairing of two hydrogen atoms, each of which possesses a single valence electron. Both atoms achieve a stable helium configuration by contributing an electron to the shared pair. Consequently, the formation of a stable H2 molecule occurs.
Each chlorine atom with the electron configuration of 2, 8, 7 possesses a total of seven valence electrons. The attainment of a stable electron octet is facilitated by the sharing of a pair of electrons between the two chlorine (Cl) atoms.
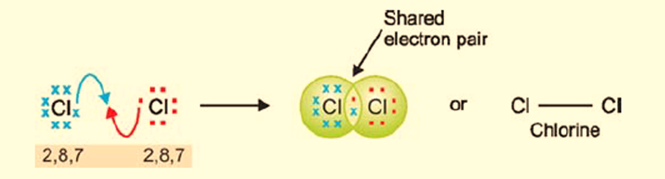
The oxygen atom with an electron configuration of (2, 6) possesses six valence electrons. It can attain a stable octet by engaging in electron sharing with two hydrogen atoms, with each hydrogen atom contributing one electron. The Lewis structure for water can be represented as:
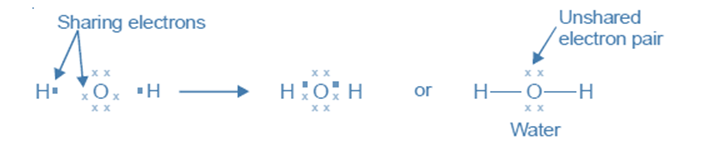
The nitrogen atom with electron configuration (2, 5) possesses a total of five valence electrons. In order to attain the octet configuration, it can engage in electron sharing with three hydrogen atoms, with each hydrogen atom contributing one electron to the shared bond. The Lewis structure of ammonia can be represented as follows:
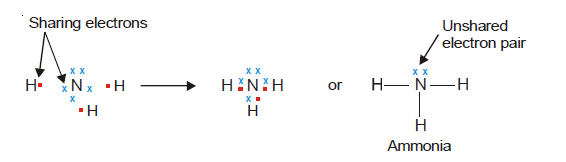
Some of the examples illustrating multiple covalent compounds are discussed below:
The Lewis structure of oxygen typically involves the sharing of two electron pairs between two oxygen atoms, each of which has an atomic number of 8 electrons in its valence shell. By doing so, both oxygen atoms attain the octet configuration.

This configuration of oxygen denotes that all electrons within the O2 molecule are paired, resulting in a diamagnetic property. Experimental evidence indicates that O2 possesses paramagnetism due to the presence of two unpaired electrons. The observed phenomenon may be attributed to the underlying structure.

While Lewis structures are effective in elucidating bonding in many uncomplicated molecules, it is important to bear in mind that they are simply a theoretical depiction. In this instance, the theoretical framework is ineffective.
The element carbon, which is located in group 14 of the periodic table and has an atomic number of 6, possesses a total of four valence electrons. The element in question engages in electron sharing with two oxygen atoms, each of which possesses six valence electrons. Consequently, the carbon atom and both oxygen atoms attain their octet configuration.

Characteristics of covalent compounds
Covalent molecules are characterized by a strong bond between the constituent atoms, which is facilitated by the sharing of electrons. However, despite this strong bond, the individual molecules are subject to weak attractive forces known as van der Waals forces. Consequently, the molecules exhibit low intermolecular forces, resulting in easy separation with minimal energy input. The following elucidates the fundamental characteristics of covalent compounds.
- Gases, liquids or solids at room temperature: Under normal circumstances, covalent compounds tend to exist as either gases, liquids, or soft solids. This phenomenon can be attributed to the relatively low strength of the intermolecular forces that exist between the constituent molecules.
- Low melting points and boiling points: In general, covalent compounds exhibit low melting and boiling points. The intermolecular forces that bind the molecules in the solid crystal lattice are relatively weak. Upon the application of heat, the molecules undergo a process of excitation and are subsequently liberated from their original positions. This results in the acquisition of kinetic energy, enabling the molecules to move freely, akin to the behavior observed in a liquid state. Due to the same underlying cause, the molecules of liquids with covalent bonds can be readily converted into a gaseous state, thus accounting for their relatively low boiling points.
- Neither hard nor brittle: Ionic compounds exhibit hardness and brittleness, whereas covalent compounds do not possess these properties. The solid crystal lattice is bound by relatively weak intermolecular forces. The molecular layer within the crystal exhibits a high degree of slip in relation to its adjacent layers and is devoid of any repulsive forces similar to those observed in ionic compounds. The crystals exhibit a low degree of cohesion and lack distinct planes of fracture when subjected to external stress.
- Soluble in organic solvents: Typically, nonpolar organic solvents such as benzene and ether exhibit a high degree of solubility towards covalent compounds. The weak intermolecular forces are readily overcome by the kinetic energy possessed by the solvent molecules. Insolubility in water is a characteristic of covalent compounds. Certain compounds such as alcohols and amines exhibit solubility in water owing to the presence of hydrogen-bonding interactions.
- Non-conductors of electricity: Due to the absence of charged ions, covalent molecules do not possess the ability to conduct electricity when in a molten or dissolved state.
- Isomerism: The covalent bonds between atoms are characterized by their rigidity and directionality, as they result from the sharing of electron pairs rather than the interaction of electrical lines of force. Various spatial arrangements can be achieved and stereoisomerism is exhibited by covalent compounds.
- Molecular reactions: Covalent compounds exhibit reactions in which the entire molecule undergoes a transformation. The absence of strong electrical forces results in a sluggish pace of molecular reactions.
Physical and Chemical properties of covalent compounds
- Covalent compounds in a liquid state undergo evaporation. This phenomenon refers to the process by which molecules from the surface of liquids and solids transition into the gaseous phase.
- These compounds exhibit low intermolecular affinity.
- Distinctive molecular structures are exhibited by diverse covalent compounds. The bonds exhibit directional characteristics at predetermined angles.
- Certain compounds, particularly those utilized in the field of medicine, exhibit solubility in aqueous solutions. The remaining substances exhibit solubility in oil.
- There are two types of crystals for covalent compounds. In certain substances, such as Iodine, the intermolecular forces, specifically the van der Waal forces, may be relatively weak, resulting in a less cohesive molecular structure. These substances exhibit high volatility and are readily fusible. The formation of macromolecules is facilitated by a vast network of atoms.
- These compounds exhibit solubility in organic solvents such as ether and benzene.
Formation of a covalent compound
The fundamental structure of an atom comprises a central nucleus that bears a positive charge, and a set of orbiting electrons that carry negative charges. The present inquiry pertains to a pair of chlorine atoms, wherein each atom contains a total of seven valence electrons. Each chlorine atom exhibits a valence electron deficiency of one in order to achieve a completed outer shell. The process of forming a covalent bond involves the sharing of a pair of electrons between two atoms. The electrons are localized within the interatomic region of the two chlorine atoms, stabilized by magnetic forces. Electrons with negative charge exhibit an attractive force towards the positively charged nucleus of each atom, thereby hindering the separation of atoms.
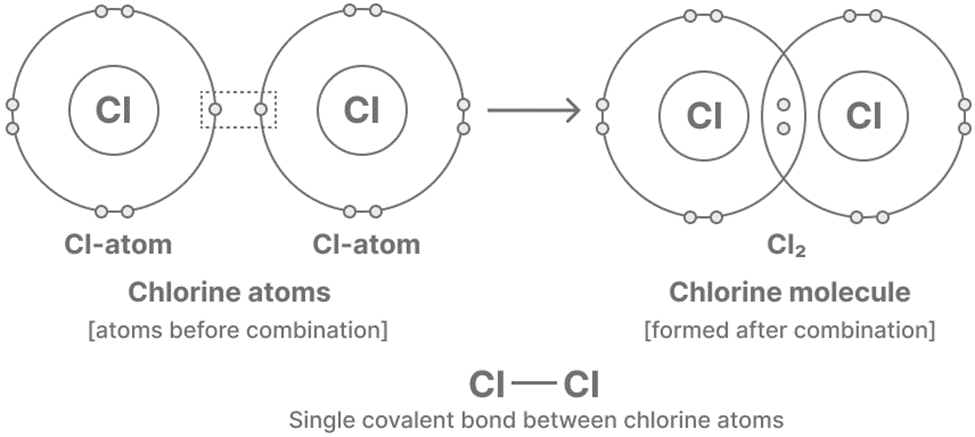
Figure: Covalent compound (https://www.knowledgeboat.com/ )
The electrons that are bonded between two chlorine atoms are distributed equally, as each atom exerts an identical force on the shared electrons. Nonetheless, this claim does not hold true universally as certain atoms exhibit greater electronegativity than others. Electronegativity is the term used to describe an atom’s capacity to attract electrons. In the case of a covalent bond between an atom with high electronegativity and another with low electronegativity, the distribution of electrons within the bond will be non-uniform. The occurrence of a dipole, which is the division of charges between two atoms that are covalently bonded, is attributed to the uneven distribution of shared electrons.
Hydrogen fluoride can be classified as a simple covalent compound that contains a dipole. The electronegativity of the fluorine atom is significantly higher than that of hydrogen. Due to the predominant localization of the shared electrons around the fluorine atom, there is an uneven distribution of charge. In the depicted image, fluorine exhibits a partial negative charge, whereas hydrogen displays a partial positive charge.
Why are covalent compounds not soluble in water?
The fundamental concept in dissolution is that of ‘like dissolves like’, whereby non-polar compounds tend to dissolve in non-polar solvents, such as hydrocarbons, due to the formation of dispersion forces between the solute and solvent. Polar solvents have the ability to dissolve polar covalent substances due to the occurrence of dipole-dipole interactions or the establishment of hydrogen bonds between the solvent and solute. Organic molecules, such as alcohols and water, serve as a prime illustration of this phenomenon.
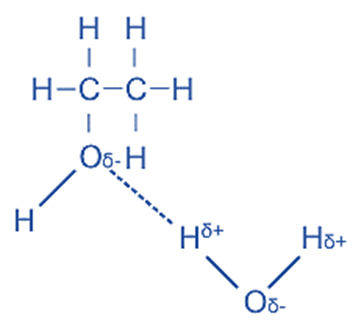
The solubility of covalent molecules may decrease with increasing size, as the polar component of the molecule constitutes a relatively smaller proportion of the overall structure. The phenomenon of varying solubility in alcohols is evident, as observed in the case of ethanol (C2H5OH) which exhibits high solubility, whereas hexanol (C6H13OH) does not dissolve easily. In general term, water molecules exhibit a degree of non-neutrality. The molecules in question exhibit a small negative charge on the oxygen atom and a minor positive charge on the hydrogen atoms. Conversely, it is understood that covalent compounds consist of either neutral molecules or molecules possessing minor charges. Consequently, these compounds exhibit weak affinity towards water molecules.
Polar covalent substances exhibit poor solubility in non-polar solvents due to inadequate interaction between their dipole-dipole attractions and the solvent. Giant covalent compounds typically exhibit insolubility in solvents due to the high energy requirement for breaking the robust covalent bonds present in their lattice structures.
Difference between covalent and ionic compound
| Covalent compound | Ionic compound | |
| Definition | Covalent compounds are formed through the sharing of electrons between two atoms. | Ionic compounds are formed through the process of electron exchange between two atoms, where one of the atoms is a metal. |
| Types of atom | Composed of two types of atoms – non-metals | Composed of metal and non-metal |
| Bond | Held together by weak intermolecular forces | Held together by strong electrostatic forces. |
| Nature | Generally found in nature as gases or liquid | Typically found in nature as solid |
| Formation | Forms when two atoms share electrons | The formation of a compound occurs through the process of electron exchange between two atoms, where one of the atoms is a metal. |
| Melting and boiling points | Have low melting and boiling points | Have high melting and boiling points |
| Structure | Held together by a network of covalent bonds | Held together by a network of ionic bonds |
| Solubility | Soluble in water | Insoluble in water |
Uses of covalent compounds
Because there is such a wide variety of covalent compounds, there is also a wide variety of applications for these molecules. The following is a small selection of the various covalent compounds and their applications for them:
- Sucrose, often known as table sugar (C12H22O11), is a popular kind of sugar that is used to sweeten dishes.
- Ammonia (NH3) is an ingredient that can be found in a variety of different types of cleaning products.
- Methane, also known as CH4, is the primary component of natural gas and has a number of applications, including home heating and gas cooking burners.
- H2O, commonly known as water, is an essential compound for the survival of all living organisms.
- Carbon dioxide is a covalent compound that finds application in the production of soft or cold drinks and other fluids, and is commonly utilized in our daily lives.
- This substance finds application in the processes of steel melting, refining, and manufacturing.
References
- Whitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond E. (1992). “7-3 Formation of covalent bonds”. General Chemistry (4th ed.).
- Pauling, L. (1960). The Nature of the Chemical Bond. Cornell University Press. pp. 340–354
- https://www.studysmarter.co.uk/explanations/chemistry/ionic-and-molecular-compounds/properties-of-covalent-compounds/
- https://www.toppr.com/guides/chemistry/chemical-bonding-and-molecular-structure/covalent-compounds/
- https://chem.libretexts.org/Courses/Mount_Aloysius_College/CHEM_100%3A_General_Chemistry_(O’Connor)/04%3A_Covalent_Bonding_and_Simple_Molecular_Compounds/4.03%3A_Covalent_Compounds_-_Formulas_and_Names#:~:text=COVALENT%20AND%20IONIC%20COMPOUNDS,-What%20elements%20make&text=Covalent%20bonds%20form%20when%20two,so%20by%20forming%20covalent%20bonds.
