Coordination compounds are complex compounds consisting of a central metal atom surrounded by nonmetal atoms or groups of atoms called ligands that are chemically linked to it.
E.g., [Co(NH3)6]Cl3, [CoCl(NH3)5]Cl2
Isomerism in Coordination compounds
Isomers are compounds that have the same chemical formula but differ in physical and chemical properties due to different structural arrangements. Due to the complicated formulae of complexes with different possible bond types and shapes, the Coordination compounds have different types of isomerism. Coordination compounds mainly consist of mainly two types of isomerism, namely structural isomerism and stereoisomerism.
Structural isomerism
This isomerism results from differences in the structures of complex (coordination) compounds. This isomerism is further divided into the following types:
Ionization isomerism
Ionization isomers are coordination compounds that have the same molecular formula but form different ions in solution. This property is known as ionization isomerism The exchange of a group between the complex ion and the ion outside it causes this type of isomerism. Red-violet [Co(NH3)5Br]SO4 and [Co(NH3)5SO4]Br are typical examples of ionization isomers. The red violet gives the sulfate ion, and the red isomer gives the bromide ion in the solution.
[Co (NH3)5 Br]SO4 ⇌ [Co (NH3)5 Br]2+ + SO42-
[Co (NH3)5 SO4]Br ⇌ [Co (NH3)5 SO4]+ + Br–
Hydrate isomerism or solvate isomerism
Hydrate isomer is similar to the ionization isomer, but it involves the exchange of solvent molecules with negative ions. The most common example of hydrate isomerism is CrCl3.6H2O, which can form three distinct crystalline compounds: [Cr(H2O)6]Cl3 (violet), [Cr(H2O)5]Cl2.H2O (blue-green), and [CrCl2(H2O)4]Cl.2H2O. (dark green).
[Cr(H2O)6]Cl3 does not lose water when stored over Conc. H2SO4. Its molar conductance is of the same order as that of trivalent salt, indicating the presence of tripositive complex ion and three mononegative chloride ions.
[Cr(H2O)5]Cl2, when stored over conc. H2SO4 loses one mole of water. It has the molar conductance of a bivalent state, indicating the presence of a dipositive complex ion and two chloride ions.
Linkage isomerism
The coordination of an ambident ligand (a ligand with more than one atom that can donate an electron pair) to the central metal ions gives linkage isomerism. Ligands like NO2–, SCN–, and CN– can coordinate with the central metal atom through either of their two donor atoms, so there is the possibility of forming two different complex compounds.
M-SCN thiocyanato isomer
M-NCS isothiocyanato isomer
M-NO2 nitro isomer
M-ONO nitrito isomer
M-CN cyanato isomer
M-NC isocyanato isomer
For example, [Co(NH3)5NO2]Cl2 has two isomers containing one single NO2– ligand in the complex ion. The Nitritro complex is red in color. It is easily decomposed by acid to give nitrous acid. In contrast, the nitro complex is yellowish-brown in color. It is stable to acid to give nitrous acid.
[Co(NH3)5ONO]Cl2 Pentamminenitritocobalt(III) ion Red
[Co(NH3)5NO2]Cl2 Pentamminenitrocobalt(III) ion Yellow-brown color
Coordination isomerism
Complex compounds containing both complex cations and complex anion show coordination isomerism. Coordination isomers are structural isomers that have both cationic and anionic parts as complexes with different ligand and/or metal ions. Coordination isomers are obtained when some or all ligands of both coordination spheres are interchanged with each other. In coordination complexes, the central metallic atom in two coordination spheres ( complex cation and complex anion) may be the same or different. Examples:
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
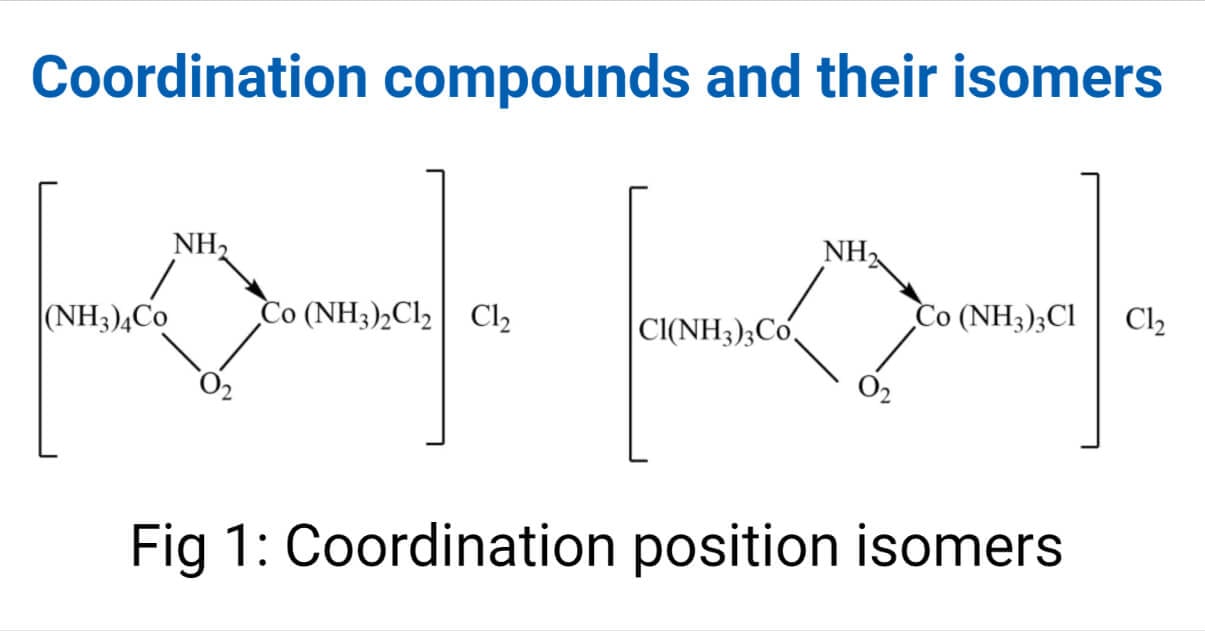
Coordination position isomerism
A special type of coordination isomerism shows by a complex containing a bridging ligand. The isomerization occurs due to the different distribution of nonbridging ligand around the central metal ion.
Example
Ligand isomerism
Certain ligands can be incorporated into complexes to rise to isomers called ligand isomers. The complexes of isomeric ligands are ligand isomers. For example, there are two isomers of composition C3H6(NH2)2 namely 1,2-diaminopropane and 1,3-diaminopropane. 1,2-diaminopropane is also called propylenediamine (pn) and 1,3-diaminopropane is known as trimethylene diamine (tn).
[Co(pn)Cl2]Cl and [Co(tn)Cl2]Cl
Polymerization isomerization
This type of certain complexes which molecular compositions that are multiples of simple complexes. The molecular formula of these compounds appears to be polymers of some simple, complex compound. E.g
[Pt(NH3)2Cl2], [Co(NH3)2Cl2], [Co(NH3)6] [CoCl6],
Stereoisomerism
The isomers that differ in the arrangement of ligands in space about the central metal ions are stereoisomers. There are two types of stereoisomerism: geometrical isomerism and optical isomerism.
Geometrical isomerism
Geometrical isomerism in four coordinate complexes
There are two geometries for the compounds having coordination numbers. These are two-dimensional square planar and three-dimensional tetrahedral geometry.
Tetrahedral complexes
In 4- coordinated tetrahedral complexes, all the coordinating positions are cis to each other, so tetrahedral complexes with monodentate ligands a, b, c, and d of all types, namely [Ma4], [Ma2b2], [Ma3b], and [Mabcd], can only exist in one geometrical form. There is no geometrical isomerism in tetrahedral complexes.
Square planar complexes
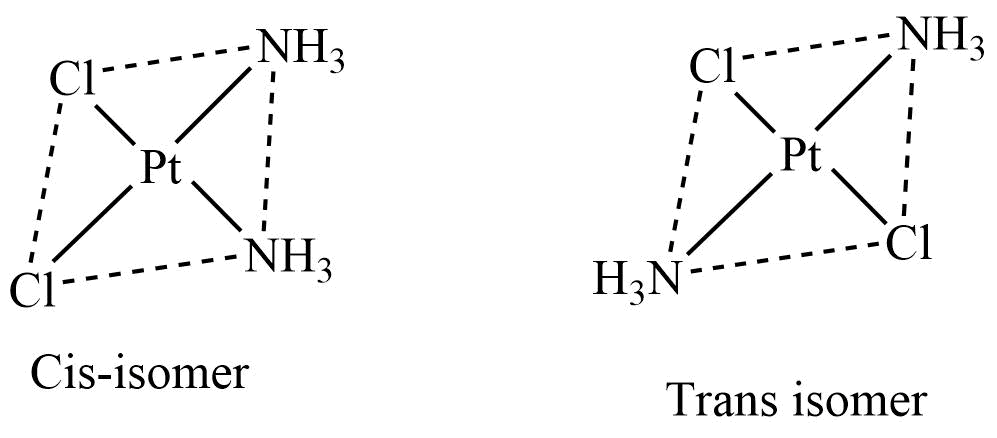
- Ma2b2 type
Square planar complexes of Ma2b2 type, where M is a central metal ion, and b are monodentate ligands, exhibit cis and trans isomers. The complexes like [Pt(NH3)2Cl2], [Pd(NH3)2(NO3)2] and [Pt(NH3)2(NO2)2] show cis-trans isomerism.
2. Ma2bc type
In this type of square planar complex, a is a neutral monodentate ligand like NH3, and H2O, while b and c are the anionic monodentate ligands such as Cl–, Br–, and NO2–. These complexes exhibit cis-trans isomerism, where isomers are distinguished on the relative position of ligand a.
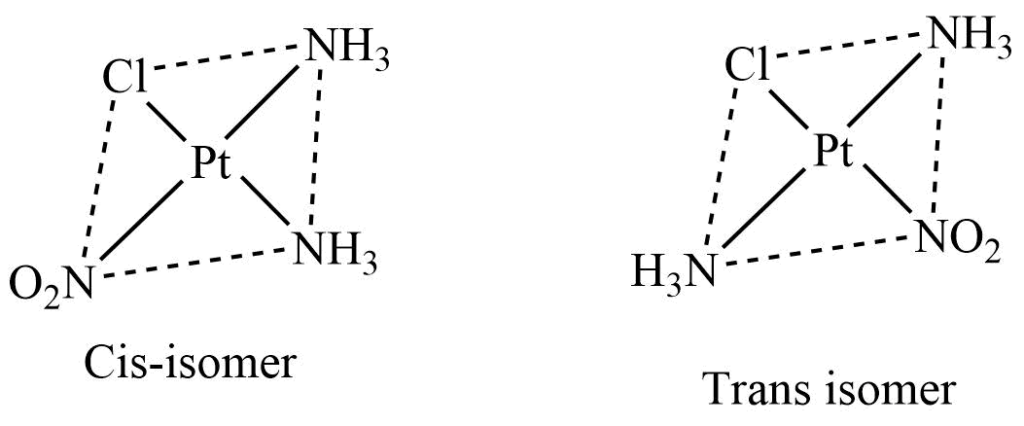
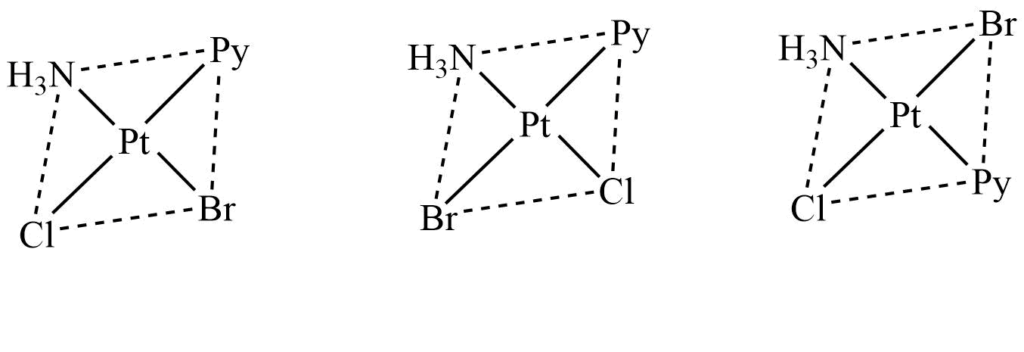
3. Mabcd type
Square planar complexes of this type exhibit three isomeric forms. These isomers can be obtained by fixing the position of ligand a and putting other ligands b,c, and d, one by one, tarns to ligand a.
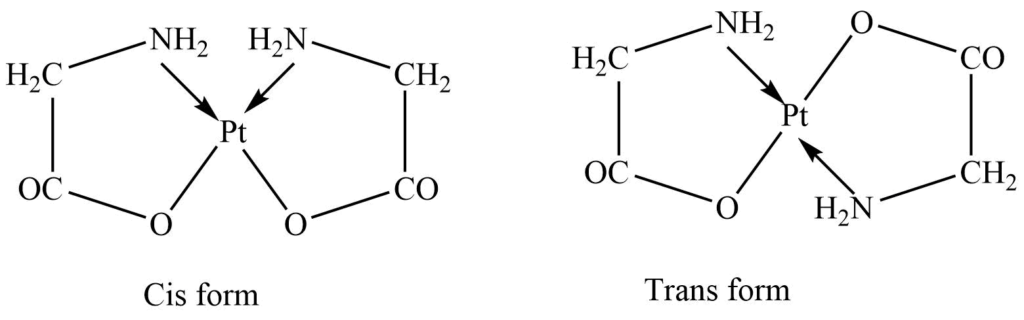
4. M(AB)2 type
M(AB)2 type complexes where AB is an unsymmetrical, bidentate ligand exist cis and trans isomers. For example [Pt(gly)2] exists as cis-trans isomers.
5. Ma4 and Ma3 b type
Square planar complexes of types Ma4 and Ma3 b, do not show geometrical isomerism, since all the possible spatial arrangement of four ligands around the central metal atom is the same.
Geometrical isomerism in six-coordinate complexes
Most metals form at least one six-coordinate complex. In these complexes, three different arrangements of six-monodentate ligands around the central metal ion are possible. These three arrangements result in different geometries, such as hexagonal planar, trigonal prismatic, and octahedral. However, the most preferred arrangement of six ligands in a complex is always octahedral, with all equivalent six positions.
Octahedral complexes
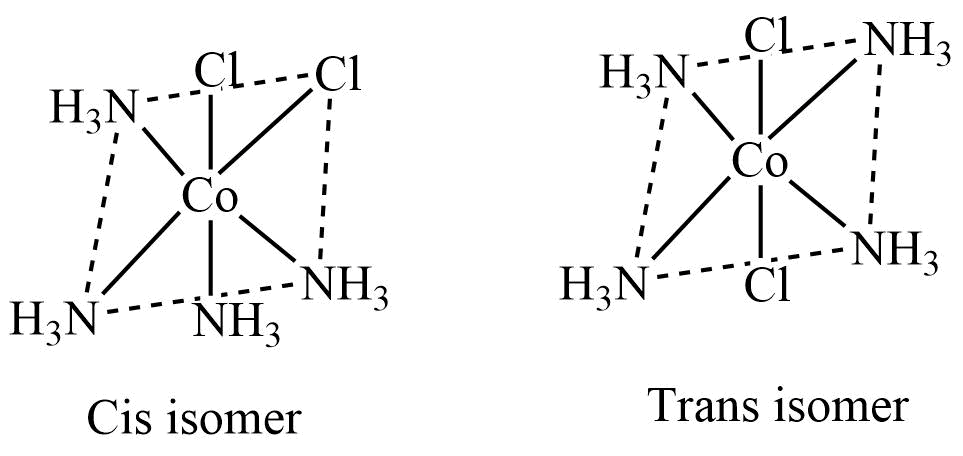
- Ma4b2 type
Octahedral complexes of Ma4b2 type, where a and b are monodentate ligands, exist as cis and trans isomers.
2. Ma3b3 type
This type of octahedral complex also exists in cis and trans isomers. In these complexes, the total number of ligand arrangements is equal to the total number of ways in which the three ligands ‘a’ can be arranged in an octahedron. [Co(NH3)3Cl3], [Cr(NH3)3Cl3], [Rh(py)3Cl3], [Ru(H2O)3Cl3] etc are the common examples of octahedral complexes of Ma3b3 type.
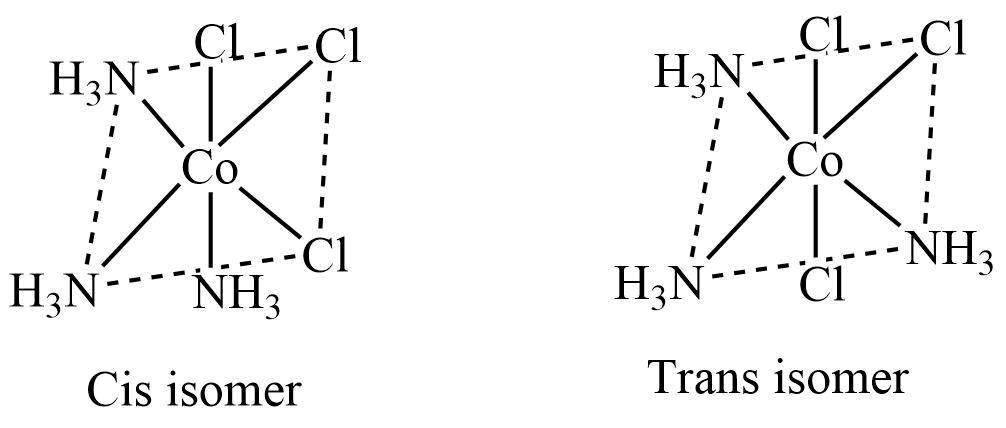
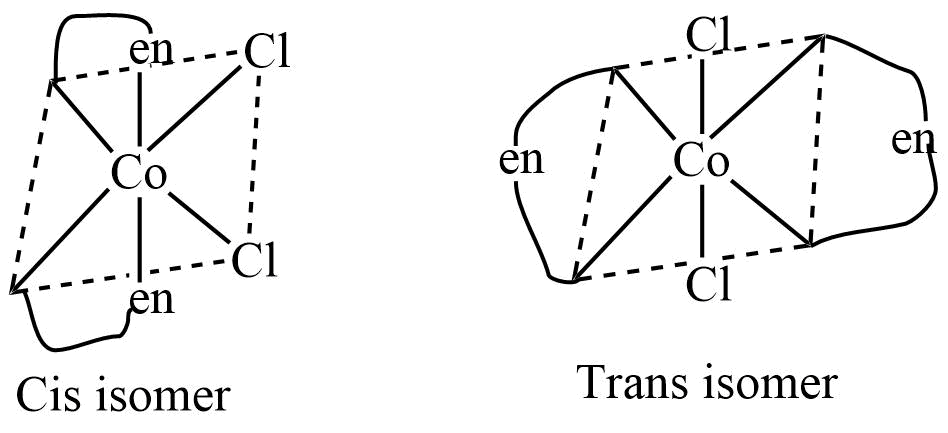
3. M(AA)2a2 type
These complexes AA unsymmetrical bidentate ligands while a is a monodentate ligand. In the cis isomer of these complexes, AA is cis to each other, while in the trans isomer, AA is trans to each other.
4. Ma2b2cd type
Ma2b2cd type octahedral complexes exist in cis and trans isomers in which the relative position of ligands a and b determine the possible isomers.
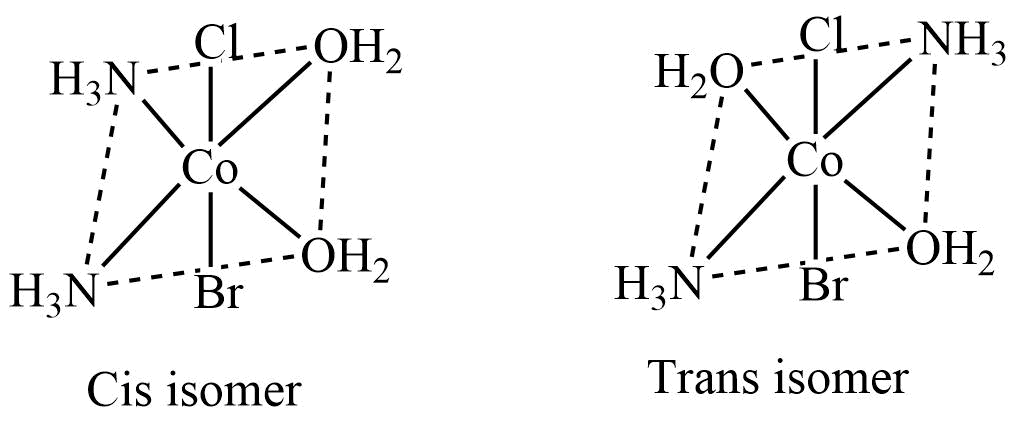
Optical isomerism
Solutions of certain complexes rotate the plane of polarized light either in a clockwise direction or in an anticlockwise direction. These complexes are optically active. The optical isomers are similar in their chemical and physical properties, but they differ in the direction of rotation of plane-polarized light.
Conditions for optical isomerism
- Molecules should be asymmetric.
- The object and its mirror image should be non-superimposable over each other.
Identification of isomeric metal complexes
Measurement of conductivity
The isomeric metal complexes constitute a different number of ions then. They would have different molar conductance. For example [Cr(H2O)6Cl3] and [Cr(H2O)5Cl]Cl2 . H2O will possess 4 and 3 ions, respectively, so they have different conductance.
Electrolysis
The heterometallic isomers (i.e one metallic ion consists of a cation complex and the other an anionic complex) can be distinguished by electrolysis. For example
I. [Co(NH3)6] [Cr(NO2)6] is isomeric with II. [Cr(NH3)6] [Co(NO2)6]
On electrolysis complex I, cobalt metal will be deposited at the negative electrode, whereas on electrolysis of complex II chromium gets deposited at the negative electrode.
Depression in freezing point
As the isomeric metal complexes consist of a different number of ions, they produce different depressions at the freezing point for a solvent.
Dipole moment
Cis and trans isomers can be distinguished cis and trans isomers. The bond moments in trans isomers are equal and opposite, so the trans isomers have zero dipole moments. The high value of the dipole moment indicates the presence of the cis isomer.
Infrared spectroscopy
Infrared absorption bands of metal complexes are due to changes in the dipole moment of the molecule. Vibrations that do not cause a change in the dipole moment do not produce bands in the IR spectra. The symmetrical stretching of trans isomers does not change the dipole moment, so they do not show IR absorption bands. Whereas in cis isomer, both symmetrical and unsymmetrical vibrations produce changes in the dipole moment, so they show different IR bands.
Linkage isomers contain the same ligand bonded differently to the metal, showing different infrared absorption frequencies. For example [Co(NH3)5ONO]Cl2 and [Co(NH3)5NO2]Cl2 . In the nitrito complex, a metal-oxygen bond is present, whereas, in the nitro complex, there is the presence of a metal-nitrogen bond. So they show different absorption frequencies in the infrared region.
NMR spectroscopy
The isomeric complexes containing hydrogen atoms in the different chemical environments would produce different H-NMR signals. For example, cis [Pt(NH3 O4 Cl2)4Cl2] and trans [Pt(NH3)4 Cl2].
Mass spectroscopy
The structural isomers form different ions with different m/z values which can be identified by mass spectroscopy.
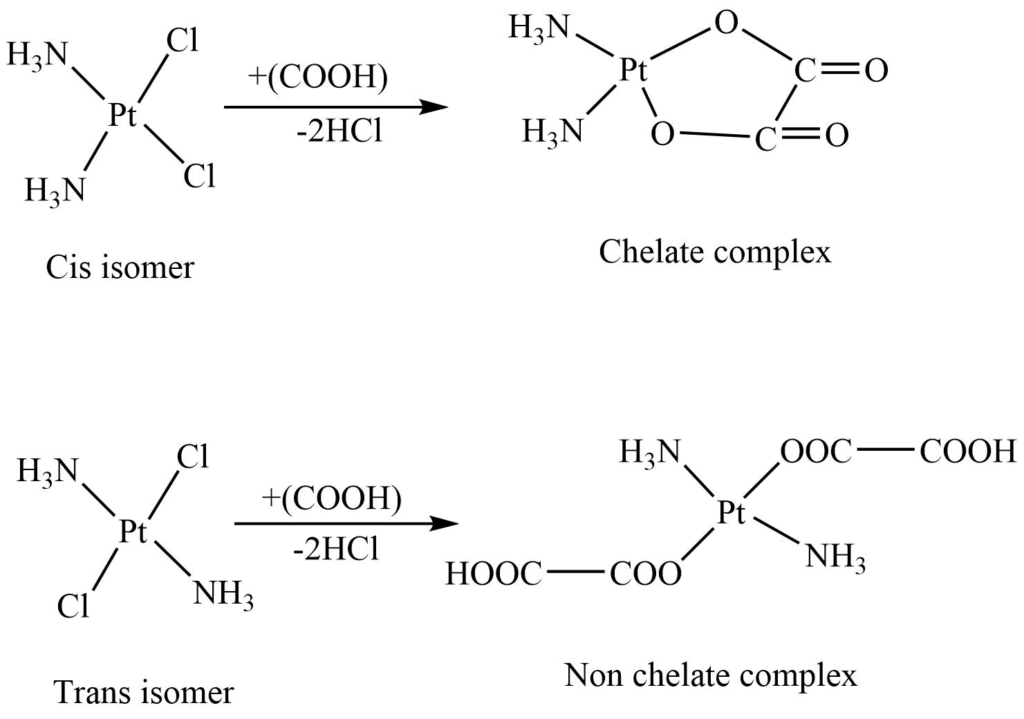
Chemical method: Grinberg’s method
The cis and trans isomers of the complex react with oxalic acid under identical conditions to give different final products. Hence by analyzing the final product, we can identify the isomers. The difference in the final products is because oxalic acid can easily occupy the cis position. Hence, it behaves as a bidentate ligand, whereas this bidentate ligand can not occupy the trans positions, so it behaves as a monodentate ligand with a trans isomer.
Factors affecting the stability of metal complex
The stability of metal complexes mainly depends upon the nature of the central metal ion, the nature of ligands, and the chelate effect, along with other factors.
The nature of central metal ion
a. Charge and size
A metal ion with a higher charge and a smaller size form a stable complex. Smaller size allows ligands to approach more closely, and higher charge results in greater electrostatic attraction, resulting in the formation of a more stable complex.
b. Electronegativity of central metal ion
The highly electronegative central metal ion strongly attracts electron pairs from the ligand, resulting in the formation of a more stable complex.
Nature of ligand
a. Charge and size of the ligand
Anionic ligands with higher charges and smaller sizes form a more stable complex. The smaller the ligand, the closer it can approach the metal, while the larger the charge, the higher the electrostatic attraction. As a result, more stable complexes are formed.
b. Dipole moment of ligands
A neutral ligand forms a stable complex with a higher dipole moment.
c. Basic character of ligands
The more basic the ligand, the more easily it can donate an electron pair to the central metal ion and thus form a more stable complex.
Chelate effect
Metal complexes with chelate rings are more stable than similar complexes. For example, complexes of ethylenediamine are more stable than those of ammonia. The extra stability of the complex with a chelate ring is called the chelate effect.
Crystal field stabilization energy
The compounds with higher crystal field stabilization energy are more stable than compounds with low crystal field stabilization energy.
References
- https://www.sciencedirect.com/science/article/pii/B9780128038956000033
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/24%3A_Complex_Ions_and_Coordination_Compounds/24.04%3A_Isomerism
- https://www.chem.uci.edu/~lawm/11-16.pdf
- http://web.uvic.ca/~djberg/Chem324/Chem324-5OUTLINE.pdf
- https://uomustansiriyah.edu.iq/media/lectures/6/6_2018_11_10!08_18_38_PM.pdf
- https://www.britannica.com/science/coordination-compound/Isomerism
- https://byjus.com/jee/coordination-compounds/
- https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s27-04-coordination-compounds.html
- https://ncert.nic.in/textbook/pdf/lech109.pdf
