Column chromatography is a technique for separating a single chemical compound from a mixture that has been dissolved in a fluid. It separates substances based on the differential adsorption of compounds to the adsorbent as the compounds move through the column at various rates, allowing them to become fractionally separated. This technique is a subset of adsorption chromatography.
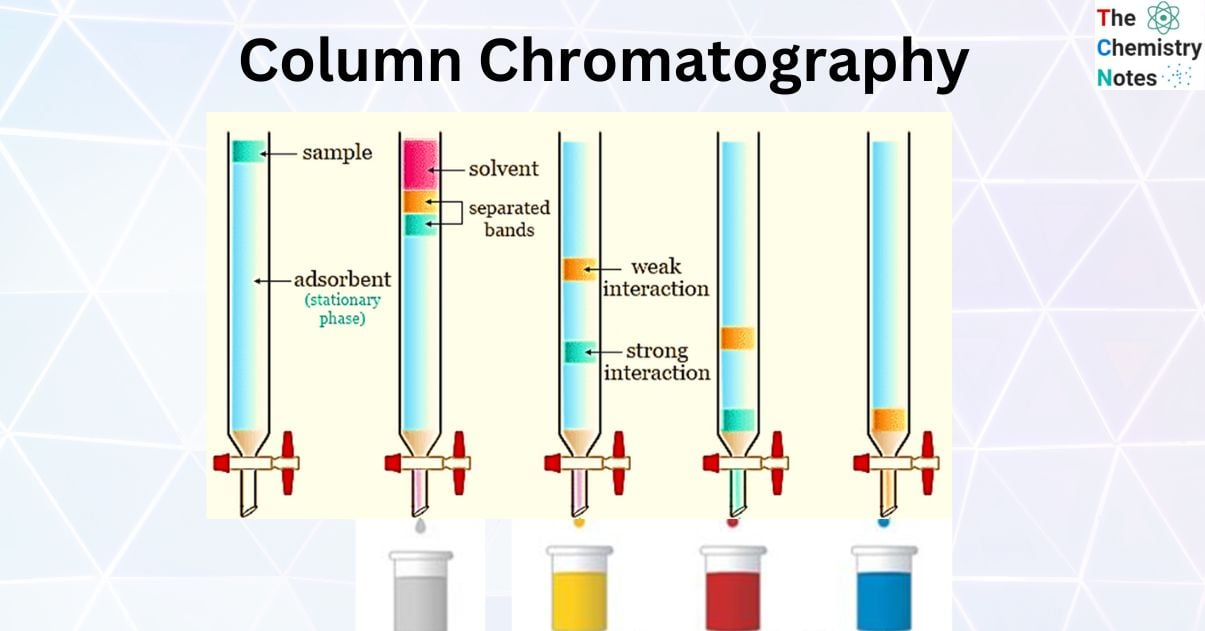
What is Column Chromatography?
Column chromatography is a useful technique that separates substances based on the differential adsorption of compounds to the adsorbent as the compounds move through the column at different rates depending on the affinities of each substance for the adsorbent and the solvent or mixture allowing them to be separated into fractions. They are typically gathered in solution as they move through the column at different times.
Principle of Column Chromatography
The principal basis of column chromatography is the solute adsorption onto a stationary phase, which separates the mixture into its component parts.
The stationary phase in column chromatography is tightly packed inside a glass or metal column. The mobile phase, also referred to as the eluent, is then passed through the column either by a pumping system or applying gas pressure after the mixture of analytes has been added. The stationary phase is either applied as a thin film to the interior of the column or coated onto discrete small particles (the matrix) and packed into the column. The analytes separate based on their distribution coefficients as the eluent passes through the column and emerge separately in the eluate as it leaves the column.
Molecules of the solute are reversibly adsorbed to the column. The following formula is used to express how quickly the components are moving:
Retardation factor (Rf) = the distance traveled by solute / the distance traveled by the solvent
Instrumentation of Column Chromatography
Column chromatography consists of a packed three-dimensional stationary phase inside the glass, plastic, or metal column and can be used for both preparative and analytical purposes.
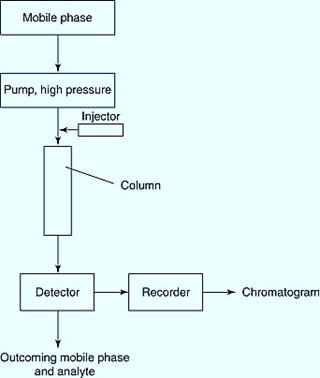
Stationary phase: In general, it is a solid material with good adsorption properties that should be suitable for the separation of analytes. It should not obstruct the flow of the mobile phase. It is chosen as the best fit for the analytes to be separated.
The mobile phase and delivery system: They are composed of solvents that complement the stationary phase. The mobile phase functions as a solvent, a developing agent (which encourages the separation of components in the sample to form bands), and an eluting agent (to remove the components from the column that are separated during the experiment).
Injector System: Tests can be accurately and repeatedly injected up to the column’s surface using an injector system.
Column: Dimensions of a column – length-to-diameter ratio (10:1, 30:1, or 100:1)
The primary function of all columns is to support the stationary phase. Because it should not be affected by solvents, the column is mostly made of high-quality neutral glass. A regular burette can also be used as a separation column. Various accessories are attached to the top and bottom of the column to keep the elution process running smoothly.
Detector and chart recorder: A continuous report of the analytes in the eluate, as it is released in the column, is provided by the detector and chart recorder. Detection is usually determined by measuring a physical parameter, such as visible or ultraviolet light absorption or fluorescence. A peak in the chart recorder represents each analyte that is separated.
Fraction collector: A device that collects separated analytes for future biochemical research.
Types of Column Chromatography
Adsorption column chromatography – Adsorption Chromatography is a separation method in which the constituents of a mixture are adsorbed onto the surface of the adsorbent.
Partition column chromatography – In partition chromatography, the stationary and mobile phases are both liquids.
Gel column chromatography – This method of chromatography separation uses a gel-filled column. It is stationary because it contains a solvent that is contained within the gaps of a solvent.
Ion exchange column chromatography – A chromatographic method in which the stationary phase is always an ion exchange resin.
Procedure of Column Chromatography
The following steps are used in the column chromatography procedure to separate a mixture of different compounds.
- Adsorbent
- Solvent selection
- Packing of column chromatography
- Sample placement
- Chromatogram development
Adsorbent
The most commonly used and commercially available adsorbents in column chromatography are activated alumina (Al2O3) and silica gel (SiO2). Activated alumina and charcoal are both effective adsorbents. Intermediate adsorbent materials include calcium carbonate, calcium phosphate, magnesia, and shake lime. The absorbability of organic compounds is determined by the nature and number of polar groups present in the compound.
Solvent selection
Adsorption is affected by the solvent used in the column chromatography procedure. A nonpolar solvent is used to place the solute in the column, but a polar solvent is used to develop the chromatogram. Poor separation results when the compounds are eluted quickly. For the slow elution procedure, we used a milder absorbent and a slightly polar solvent. As a result, we use nonpolar solvents for weakly absorbed compounds and polar solvents for strongly absorbed compounds.
The most important step in the column chromatography procedure is the polarity and solvent selection. The polarity of commonly used solvents is listed in the following order:
Petroleum ether < carbon tetrachloride < cyclohexane < carbon disulfide < ether < acetone < benzene < ethyl acetate < chloroform < ethanol < methanol < water < pyridine < acetic acid.
Packing of column
The packing of the column in the chromatography procedure is critical for avoiding air bubbles and separation errors. The column tube is thoroughly cleaned as well as dried. When running the solvent, it should not be allowed to dry until the experiment is finished. In chromatography, columns are either dry packed or wet packed.
Dry Packing
In dry packing, we used a glass tube with a stopper. Using a glass rod, some glass wool or cotton wool is placed at the top of the constricted portion of the glass tube. The glass allows the solvent to pass through while keeping the absorbent in the column, which is positioned vertically.
To set the absorbent, a weighted amount of absorbent is added to a small portion of the tube. We washed the column with eluting solvent before using it. To protect the surface of the glass tube, a disc of filter paper is therefore placed.
Wet Packing
A glass tube is clamped vertically in the wet packing of column chromatography by keeping the glass tube at the constricted end. The tube’s Haft is filled with eluting solvent. A mobile absorbent slurry made from eluting solvent. The column’s stopper is opened to allow the absorbent to settle. The procedure is repeated until the entire slurry is added. A disc of filter paper is placed on top of the surface to protect it.
Sample placement
A solvent solution or a direct liquid mixture of the sample is used in the column chromatography procedure. We used polar solvent when a solid sample was insoluble in nonpolar solvent. To prepare the thick slurry, it is poured into a small amount of adsorbent and placed in a porcelain basin. When the slurry turns power form, the solvent is completely evaporated by stirring with a glass rod.
Chromatogram development
A sample mixture solution in a suitable solvent is then allowed to slowly pass down the column. A chromatogram is displayed when the individual components are selectively absorbed. If the chromatogram’s bands are colorless, a quartz column is used instead of a glass column. It is exposed to the light of a mercury lamp in order to detect the band with fluorescence. The reagents that formed color with the components of the sample mixture can also detect colorless compounds.
Separation of Compound in Column Chromatography
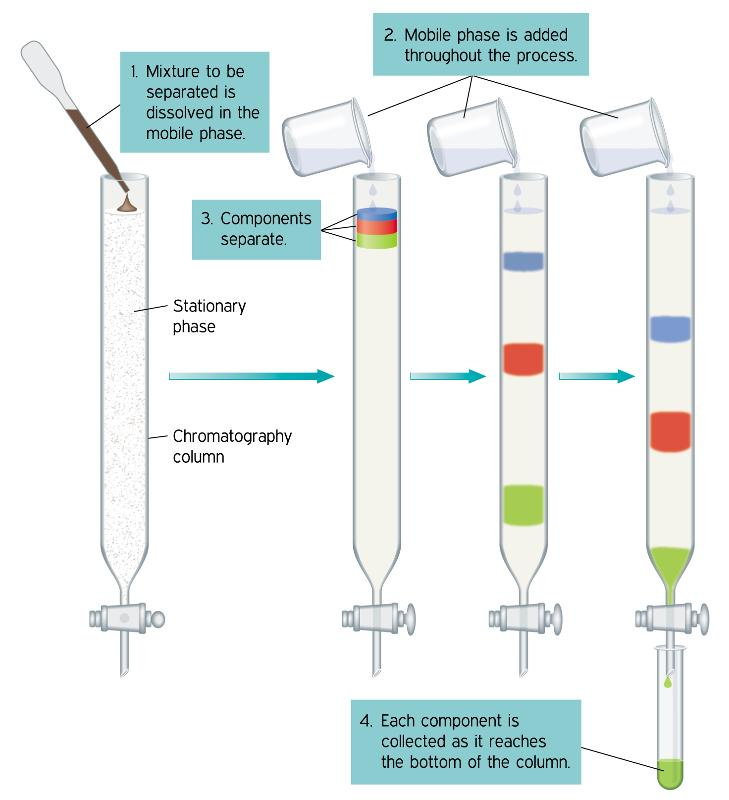
Column chromatography is accomplished in a few steps:
Step 1: Either a solvent or a solvent mixture is used as the mobile phase or eluent. The mobile phase upper level should be the same as the stationary phase. That is, the stationary phase should be soaked in the solvent. The compound mixture that needs to be separated is added from the top of the column in such a way that the top level of it is not disturbed at this stage. It is allowed to adsorbed on the surface of the silica by turning on the tap below.
Step 2: The solvent or a suitable solvent mixture is then added, slowly and carefully touching the side of the glass column so that the top level of the stationery phase is not disturbed. Throughout the process, the solvent is added as many times as necessary.
Step 3: When the tap is turned on, the compounds in the compound mixture, depending on the polarity of the sample molecule, move along with the eluent. Non-polar component’s move faster than polar components.
Step 4: If the compounds separated from the column chromatography process have a color, the separation process can be visually observed. Also, the compounds to be separated by column chromatography are colorless. In this case, small amounts of liquid are collected sequentially in tubes labeled with the composition and chemical makeup of each fraction, which is then examined using TLC.
Thus, it is used to separate or purify a compound mixture.
Advantages of Column Chromatography
- It facilitates the separation of any mixture.
- It aids in the removal of impurities from any type of mixture.
- This allows for the separation of any variety and quantity.
- Depending on the desired results, different solvents can also be used in the separation process.
- The procedure can also be automated.
- It is less costly.
Disadvantages of Column Chromatography
- It is a lengthy procedure.
- Separation requires a large amount of material.
- It is more expensive than thin paper column chromatography.
- It’s a lengthy procedure. As a result, focus and attention are required.
- It will become more expensive if the process is automated in the future.
Application/Uses
Column chromatography is one of the most effective methods for separating and purifying solids and liquids. Its primary applications include:
- Most importantly, it is used in the separation of a compound mixture.
- Impurities are removed or purified during the purification process.
- Active constituent isolation.
- Metabolites are isolated from biological fluids.
- Drug estimation in formulations or crude extracts.
Also Read: Gas Chromatography: Principle, Parts, Types, Advantages, Disadvantages
References
- Furniss, Brian S.; Hannaford, Antony, J.; Smith, Peter W. G.; Tatchell, Austin S. (1989). Vogel’s Textbook of Practical Organic Chemistry. Longman Scientific & Technical. p. 203. ISBN 978-0582462366.
- https://lab-training.com/column-chromatography/
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://www.bio-rad.com/en-np/applications-technologies/introduction-column-chromatography-methods-instrumentation?ID=MWHB7PIVK
- https://forensicfield.blog/column-chromatography/
- https://www.priyamstudycentre.com/2021/11/column-chromatography.html
- https://www.bioanalysis-zone.com/how-does-chromatography-work/
- https://en.wikipedia.org/wiki/Column_chromatography
