Colloid Definition
A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance.
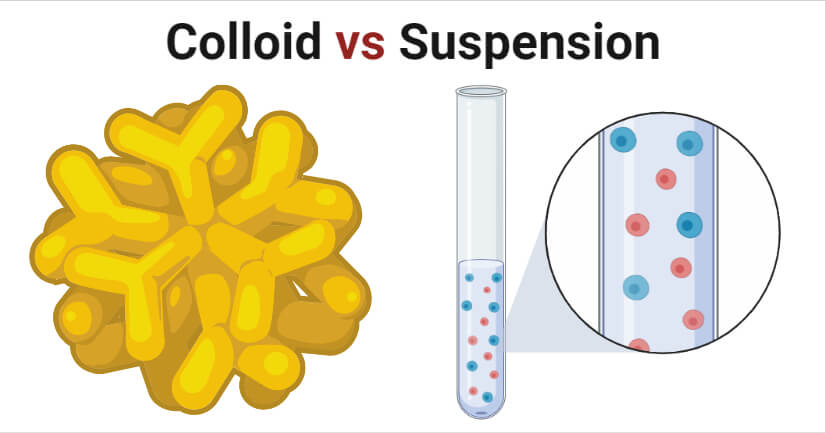
- The colloid can be differentiated from a solution in that the solute and solvent in a solution are of the same phase, but a colloid has a dispersed phase and a continuous phase as a result of the phase separation.
- Colloids usually do not settle completely and take a longer time to separate into different layers.
- Colloids contain particles that are substantially larger than atoms and molecules but still microscopic. The dimension of the particles usually ranges between 10-7 to 10-3 cm.
- Most colloids are opaque and have a slight color, but some might appear translucent as a result of the Tyndall effect.
- In general, colloids can be classified into two systems; reversible and irreversible colloids.
- In reversible systems, the products of the reactions can be induced to interact in a way to generate the original components.
- The colloidal matters in reversible systems usually have smaller molecules with small molecular weights that associate spontaneously to form larger particles.
- In an irreversible system, the products of the reactions are so stable or different that the original components of the system cannot be reproduced.
- Colloidal systems can be generated or separated by natural as well as industrial and technological processes.
- It is difficult to measure the size of the dispersed phase in colloids due to the small size, thus most colloids are identified and characterized by the physio-chemical and transport properties.
- The stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. Aggregation is one of the most important reasons for the decreased stability of colloidal systems.
- Colloids have been recently identified as a model system for atoms as the forces that govern the structure and behavior of matter are known to govern the behavior of colloidal systems.
Suspension Definition
A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent.
- The particles in suspension are larger in size, usually ranging above one micrometer. These can be observed with our naked eyes.
- The particles in a suspension eventually settle down; however, they cannot be classified as a suspension when the particles are settled down.
- The solid particles in the suspension are termed the internal phase, whereas the solvent or fluid is termed the external phase.
- The dispersion of the internal phase to the external phase is often the result of mechanical agitation with the use of suspending agents.
- Suspensions are of different types and are named differently depending on the state of the internal and external phases.
- A suspension of liquid or solid particles in a gas is called an aerosol, whereas suspension of gases in solid-like hydrogen in palladium can also be found.
- Suspensions are also classified on the basis of the state of the dispersed phase and dispersion medium. The dispersed phase is usually solid, but the dispersion medium may be solid, liquid, or gas.
- From a thermodynamic point of view, suspensions are highly unstable; however, over a long period of time, these can be kinetically stable. The stability of a suspension also determines its shelf life.
- Separation of a suspension is comparatively easy as these can be separated by simple methods by filtration and sedimentation.
- Most suspensions are opaque as these do not transmit light but cause scattering of light. Some suspensions might even exhibit the Tyndall effect.
- Suspensions are essential in pharmaceutical industries for the production of medicines and other pharmaceutical products.
12 Major Differences (Colloid vs Suspension)
| Characteristics | Colloid | Suspension |
| Definition | A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. | A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. |
| Size of the particles | Colloid particles are comparatively smaller, usually ranging in size between 10-7 to 10-3 cm. | Suspension particles are comparatively larger with sizes greater than 10-3 cm. |
| Filter paper | Colloid particles can pass through filter paper. | Suspension particles cannot pass through filter paper. |
| Visibility | The particles are microscopic and, thus, cannot be seen with our naked eyes. | The particles can be clearly seen with naked eyes. |
| Sedimentation | The particles in the colloid do not sediment as a result of gravity. | The particles in suspension undergo sedimentation as a result of gravitational force. |
| Phase separation | Phase separation is rather slow or might not even happen. | Distinct phase separation can be observed. |
| Homogeneity | Colloids are homogenous. | Suspensions are heterogeneous. |
| Diffusion | Colloids diffuse slowly. | Suspensions do not diffuse at all. |
| Optical Properties | Colloids can be either opaque or transparent, and they scatter light through them. | Suspensions are opaque as they do not transmit light. |
| Tyndall effect | Colloids demonstrate the Tyndall effect. | Suspension may or may not exhibit the Tyndall effect. |
| Uses | Colloids have industrial applications in paint, food, and various other industries. | Suspensions are essential in the production of medicines and other products. |
| Examples | Examples of colloids include milk, shampoo, foam rubber, etc. | Examples of suspension include muddy water, soot in the air, oil and water, etc. |
Examples of colloids
Milk
- Milk is a colloid consisting of microscopic dispersion of fat in the aqueous solution of plasma with dispersed lactose, other mineral salts, and colloidally dispersed proteins.
- The important colloidal proteins in milk include calcium phosphate, caseinate, and lactalbumin.
- The physio-chemical structure of milk is determined by various proteins, including colostrum which is rich in globulin.
- The content of milk proteins and fat differs in different types of milk. Cow’s milk has lesser fat content than the milk for a buffalo.
- The casein content of cow’s milk is between 2.25% to 2.75%, whereas the lactalbumin ranges between 0.5-0.7%.
- The colloidally dispersed minerals like calcium phosphate can be filtered out from milk by ultra-filters with larger porosity.
- The stability of milk is determined by the concentration of different particles in milk and various environmental factors as well.
Examples of suspension
Blood
- Blood is a suspension consisting of larger blood cells suspected in extracellular fluid material called plasma.
- It is a specialized fluid connective tissue essential for the transport of oxygen, nutrients, and water to different parts of the body.
- The plasma occupies about 55% of the total volume of blood, whereas the rest is occupied by blood cells, nutrients, respiratory gases, and waste products.
- Blood also contains plasma proteins like albumin and globulins essential to maintain the osmotic pressure and transport of various molecules.
- The blood cells can be separated with the help of centrifuges that enable the separation of plasma.
References and Sources
- Leroy S. Palmer. The Chemistry of Milk and Dairy Products Viewed from a Colloidal Standpoint. Industrial & Engineering Chemistry 1924 16 (6), 631-635 DOI: 10.1021/ie50174a041
- 4% – https://en.wikipedia.org/wiki/Suspension_%28chemistry%29
- 2% – https://en.wikipedia.org/wiki/Colloid
- 1% – https://www.toppr.com/guides/chemistry/surface-chemistry/colloids/
- 1% – https://www.thoughtco.com/definition-of-suspension-605714
- 1% – https://www.sciencedirect.com/science/article/pii/B9780444632838000259
- 1% – https://vivadifferences.com/difference-between-colloid-and-suspension-with-examples/
- 1% – https://quizlet.com/1048858/chapter-13-the-blood-flash-cards/
- 1% – https://microbenotes.com/connective-tissue/
- 1% – https://ij-chemistry.blogspot.com/2009/11/colloid.html
- 1% – https://en.wikipedia.org/wiki/Coloid
- 1% – https://brainly.in/question/19451016
- 1% – http://navier.engr.colostate.edu/whatische/ChEL05Body.html
- <1% – https://www.meritnation.com/ask-answer/question/what-is-the-difference-between-suspension-and-colloidal-solu/is-matter-around-us-pure/1262299
- <1% – https://www.homestratosphere.com/types-of-milk/
- <1% – https://www.britannica.com/science/serum-albumin
- <1% – https://www.britannica.com/science/colloid
- <1% – https://glosbe.com/en/en/external%20phase
