A colligative property is an important concept in chemistry. The term “colligative” was derived from the Latin word “colligatus,” which means “bound together.” Colligative properties explain how the properties of a solution are related to the concentration of a solute in the solution. Colligative properties are the physical changes that occur when a solute is added to a solvent. Colligative Properties are affected by the number of solute particles present as well as the amount of solvent present, but not by the kind of solute particles, however, they are affected by the type of solvent. It is also known as additive property.
What is Colligative Properties?
A colligative property is a property of a solution that is determined by the ratio of the total number of solute particles (in the solution) to the total number of solvent particles.
The chemical nature of the particles of the solution does not affect colligative properties.
Colligative properties are most commonly observed and studied in dilute solutions. Colligative properties may therefore be related to several parameters that describe the concentration of a solution, including molarity, normality, and molality.
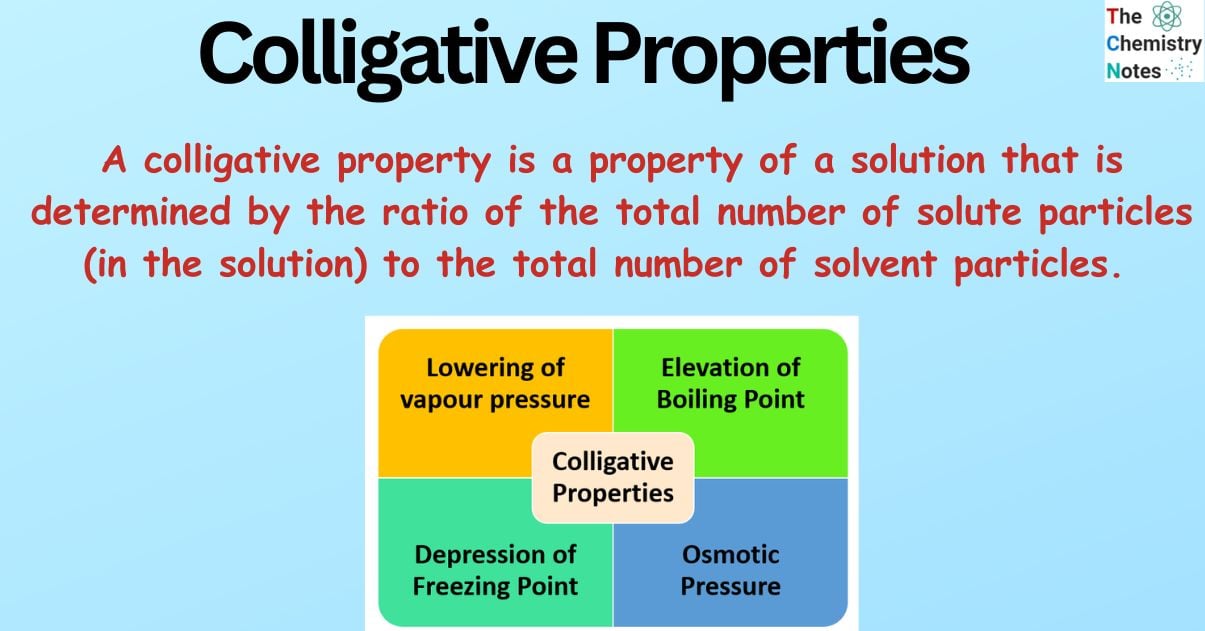
The following are the four colligative properties that a solution can exhibit:
- Lowering of vapour pressure
- Elevation of Boiling Point
- Depression of Freezing Point
- Osmotic Pressure
Colligative Properties Examples
The following examples can be used to observe the colligative properties of solutions.
- A glass of water’s freezing temperature will drop significantly below average if we add a pinch of salt to it. Alternately, the solution will have a lower vapour pressure and its boiling temperature will likewise rise. The osmotic pressure of it will also alter.
- Similarly, when we mix alcohol with water, the freezing point of the solution falls below the typical temperature for either pure alcohol or water.
- You may have noticed that adding salt causes the ice to melt; this is the standard method for de-icing snow-covered roadways. The most popular types of salt used to de-ice roadways are calcium chloride and sodium chloride. A mix of water and dissolved salt has a lower freezing point than pure water due to a depression in the freezing point of a solution, which is the major reason to sprinkle salt on an ice road. When we add salt, the temperature of the water, which ordinarily freezes at 32°F, decreases. The freezing point of a substance decreases with salt content. This is another important example of colligative properties.
Types of Colligative Properties
A solution can have a variety of colligative properties such as vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. Let us discuss each types of colligative properties in detail.
Lowering of Vapour Pressure
When a non-volatile solute is introduced to a solvent, some of the solute molecules take up surface space and slow the solvent’s rate of evaporation. These solutes are not easily ejected since they are non-volatile. These phenomena lower the vapor pressure of the solution. In such instances, the vapor pressure of the solution is always lower than that of the pure solvent. This lowering in vapor pressure is proportional to the quantity of non-volatile solute added to the solution, regardless of its type, and is thus one of the colligative properties.
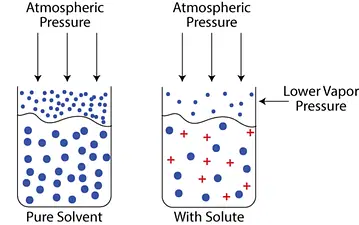
How does addition of solute lowers the vapor pressure?
Consider surface tension while understanding vapor pressure lowering. A liquid’s surface exhibits adhesion and cohesion among its molecules. Adhesion is a result of Van Der Waal interactions. The surface molecules must overcome Van Der Waal interactions in order to evaporate. The electrostatic interactions increase when a solute is added. Therefore, a molecule needs more energy to transition into the gas phase.
Let’s think about water, the universal solvent. Hydrogen bonds in pure water lead to adhesion. What occurs when sodium chloride (NaCl) or another ionic salt is added?
Ions form when the ionic bond breaks down. On the surface of the solution, some ions will be present. So, the number of water molecules that may evaporate on the surface is decreased. Additionally, some of the water molecules are now involved in ion-dipole interactions. Compared to hydrogen bonds, they are stronger. As a result, the water is more difficult to evaporate. The vapor pressure is now lower.
Elevation of Boiling Point
When a substance is added to a liquid, such as a solvent, its boiling point rises, causing the solution to have a higher boiling point than the solvent alone. This process is known as boiling point elevation. When a non-volatile solute is introduced to a pure solvent, the boiling point rises. The boiling point elevation is a colligative property, meaning that it is influenced by the presence and number of dissolved particles but not by their identity. When we take an electrolyte solute, such as various salts, or a nonelectrolyte, the boiling point rises.
How does addition of solute increase the boiling point?
A liquid must have a higher vapor pressure than the surrounding atmosphere in order to boil, which is more challenging to do when a nonvolatile component is present. If you prefer, you might conceive of adding a solute as diluting the solvent.
Because the addition of a non-volatile solute lowers a solution’s vapour pressure, the boiling point of a solution is higher than that of the solvent, requiring it to be heated to a higher temperature to obtain a vapour pressure equal to atmospheric pressure. As a result, the solution boils more quickly than the pure solvent.
Depression of Freezing Point
The addition of solutes lowers the freezing point of solvents, which is referred to as a depression of freezing point. The freezing point of the solution is lower than the freezing point of the pure solvent. When another substance is dissolved in a pure liquid, its freezing point drops. It is a colligative property, which means that the freezing point decreases according to the number of dissolved particles (molecules or ions), regardless of the nature of those particles.
How does addition of solute lowers the freezing point?
There is an equilibrium between the liquid and solid states of a solvent at its freezing point. This means that the vapor pressures of the liquid and solid phases are equivalent. The vapor pressure of the solution is found to be lower than the vapor pressure of the pure solvent after the addition of a non-volatile solute. This causes the solid and solution to attain equilibrium at lower temperatures.
Osmotic Pressure
Osmotic pressure, a third colligative property, helped to establish the basics of modern physical chemistry and was especially crucial in the early days of solution theory. Osmosis is particularly essential in medicine and biology, but in recent years it has also been used industrially for purposes such as fruit juice concentration, saltwater desalting, and municipal sewage purification.
If more pressure is applied from the solution side, the solvent molecules will not pass through the semipermeable barrier. The osmotic pressure of the solution is the force preventing solvent flow.
Osmotic pressure develops due to addition of solutes in a solvent. More the concentration of solutes in a solution, greater will be the osmotic pressure developed.
References
- Atkins, Peter and de Paula, Julio. Physical Chemistry for the Life Sciences. New York, N.Y.: W. H. Freeman Company, 2006. (124-136).
- Laidler, K.J.; Meiser, J.L. (1982). Physical Chemistry. Benjamin/Cummings. ISBN 978-0618123414
- T. Engel and P. Reid, Physical Chemistry (Pearson Benjamin Cummings 2006
- Tro, Nivaldo J. (2018). Chemistry: Structure and Properties (2nd ed.). Pearson Education. ISBN 978-0-134-52822-9.
- McQuarrie, Donald, et al. Colligative properties of Solutions” General Chemistry Mill Valley: Library of Congress, 2011. ISBN 978-1-89138-960-3.
