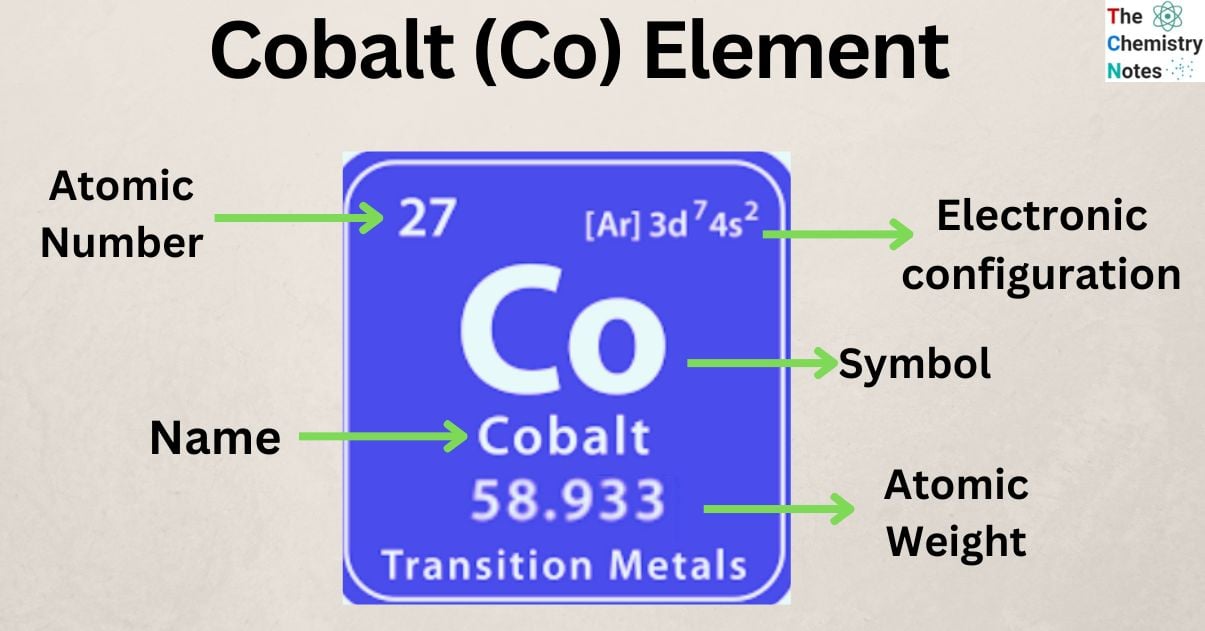
Cobalt is a chemical element with the atomic number 27 and is represented by the symbol ‘Co’ in the periodic table. It is classified as a transition and belongs to group 9 (IX) of the periodic table.
Cobalt (Co) is a white, brittle, hard metal that is similar to nickel (and iron) in appearance but has a bluish tint rather than the yellow of nickel. Cobalt does not occur naturally as a free element. Cobaltite (CoAsS), erythrite (cobalt hydrated arsenate), glaucodot (Co,Fe)AsS, and skutterudite (Co,Ni)As3 are the primary cobalt ores.
Interesting Science Videos
History of Cobalt
- Cobalt compounds have been used to make blue glass and pottery since ancient times.
- Compounds of cobalt are being used for imparting rich blue color to glass, glazes, and ceramics. Traces can still be found in the sculptures of Egyptians to the jewelry of Persian from the third millennium BC. They are found in the ruins of Pompeii which was destroyed by the supervolcano in 79 AD.
- Chinese artifacts dating back to Tang dynasty(618 AD -907 AD) and Ming dynasty (1368 AD-1644 AD) are discovered which proves the use of cobalt compounds in ancient civilizations.
- The word “cobalt” comes from the German word “Kobalt,” which comes from the term “goblin” (kobold), which was the superstitious name that miners gave to the cobalt ore.
- In the year 1735, Swedish chemist George Brandt isolated cobalt for the first time. He demonstrated that contrary to what had been thought it was actually the element cobalt, rather than bismuth, that was responsible for the blue hue of glass.
- He identified the previously unknown element and reported his discovery in 1739, and despite initial disagreement, cobalt was eventually recognized by the scientific community as a chemical element.
Occurrence of Cobalt
- Cobalt has an abundance of 30 parts per million, or 0.003% by mass, in the earth’s crust.
- Free cobalt (the native metal) is not found on Earth because of the oxygen in the atmosphere and the chlorine in the ocean.Both are abundant enough in the upper layers of the Earth’s crust to prevent native metal cobalt from forming.
- According to some estimates, world reserves of cobalt are at 7,100,000 metric tons. The Democratic Republic of the Congo (DRC) currently produces 63% of the world’s cobalt.
- A huge reserve of several transition metals (including cobalt) can be found in strange nodules on the floors of the deepest oceans. The nodules are manganese minerals that take millions of years to form, and together they contain many tonnes of cobalt.
- DR Congo, Canada, Australia, Zambia, and Brazil all have significant mineral resources. Most cobalt is produced as a by-product of the processing of nickel.
Cobalt is a chemical element that can be found in a wide range of minerals that have different mineralogical properties. Several of the typical cobalt-containing minerals are:
- Cobaltite (CoAsS)
- Erythrite (Co3(AsO4)2· 8H2O)
- Smaltite (CoAs2)
- Carrollite (Cu(Co,Ni)2S4)
Cobalt is primarily formed through geological processes associated with the formation of ore deposits. The exact formation mechanisms of cobalt deposits can vary depending on the specific type of deposit, but some common processes involved in cobalt formation include:
- Magmatic processes
- Hydrothermal processes
- Sedimentary processes
- Lateritic weathering processes
- Supergene processes
The geology, geochemistry, and tectonic context of the deposit, as well as other variables, can all affect the exact formation process of cobalt deposits, which can be complicated. Understanding the formation processes of cobalt deposits is critical for mineral exploration and mining operations.
Isotopes of Cobalt
Naturally occurring cobalt consists of a single stable isotope 59Co.
There are 22 radioisotopes that have been classified, with 60Co, 57Co, 56Co, and 58Co having the longest half-lives (5.2714 years, 271.79 days, 77.27 days, and 70.86 days, respectively). The half-lives of all the remaining radioactive isotopes are all less than 18 hours, with the majority of them being less than 1 second.
Elemental Properties of Cobalt
| Electronic Configuration | [Ar] 3d7 4s2 |
| Atomic Number | 27 |
| Atomic Weight | 58.933 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 9, 4, d-block |
| Density | 8.9 g.cm -3 at 20 °C |
| Ionic radius | 0.078 nm (+2) ; 0.063 nm (+3) |
| Van der Waals radius | 0.125 nm |
| Electron shells | 2, 8, 15, 2 |
| Electrons | 27 |
| Protons | 27 |
| Neutrons in most abundant isotope | 32 |
Physical Properties of Cobalt
- Cobalt has an atomic number of 27 and is a lustrous silvery-blue metal.It has a melting point of 1495 °C (2723 °F) and a boiling point of 2927 °C (5301 °F).
- The density of cobalt is 8.9 grams per cubic centimeter.
- It is recognized as a ferromagnetic element. ‘Co’ is one of only three metal elements that are naturally magnetic (the others being iron, Fe, and nickel, Ni).
- Cobalt is ductile (it can be pulled out into thin wires) and malleable to some extent (it can be pressed into thin sheets).
- Cobalt serves as an excellent electrical conductor. Because electrons in cobalt are free to move around they are able to carry electrical charge from one end to other.
- Cobalt is an excellent thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- It is inert in air and does not react with water but with dilute acids, it reacts slowly.
- Cobalt is odorless and tasteless.
| Color/physical appearance | Bluish white |
| Melting point/freezing point | 1495°C, 2723°F, 1768 K |
| Boiling point | 2927°C, 5301°F, 3200 K |
| Density | 8.9 g.cm-3 at 20°C |
| Malleability | Yes |
| Ductility | Somehow |
| Electronegativity | 1.88 (Pauling Scale) 1.84 (Allen Scale) |
Chemical Properties of Cobalt
Cobalt is a moderately reactive element. It reacts with oxygen in the air but does not catch fire or burn unless in powder form.Cobalt can react with most acids to produce hydrogen gas.
However, cobalt does not react with room-temperature water.
Chemical Reaction of Cobalt
- Reaction Of Cobalt With Air
Cobalt does not seem to show reactive properties with air. However, when cobalt is heated Co3O4 oxide is formed. when the reaction is performed above 900°C it results in cobalt(II) oxide, CoO. It doesn’t react with nitrogen, N2.
3Co(s) + 4O2(g) → 2Co3O4(s)
- Reaction Of Cobalt With Water
Water doesn’t seem to have much effect on cobalt. but, the reaction that occurs between red hot cobalt and steam produces cobalt(II) oxide, CoO.
2Co(s) + O2(g) → 2CoO(s)
- Reaction Of Cobalt With Acids
Cobalt metal slowly dissolves in diluted sulfuric acid to produce solutions that comprise hydrogen gas (H2) and the ionized Co(II) ion. The complex ion [Co(OH2)6]2+ represents the Co(II) in practice.
Co(s) + H2SO4(aq) → Co2+(aq) + SO42-(aq) + H2(g)
- Reaction Of Cobalt With The Halogens
The direct reaction between cobalt metal and bromine (Br) results in the production of dibromide cobalt(II) bromide, CoBr2.
Co(s) + Br2(l) → CoBr2(s) [green]
Although it is possible to create the corresponding chloride (Cl) and iodide (Id) in an identical manner, different procedures appear to be preferred for synthetic use.
Co(s) + Cl2(g) → CoCl2(s) [blue]
Co(s) + I2(s) → CoI2(s) [blue-black]
Uses Of Cobalt
One of the most sought-after and used metals in contemporary industry is cobalt. There are many other uses for it, but only a handful are covered below.
Used As Superalloy
Cobalt is being used to make a superalloy which is a kind of high-temperature metal material that has worked for a long time above 760 ~ 1500 ℃ under certain stress condition. This material comprises high-temperature strength, outstanding oxidation and heat corrosion resistance, good fatigue capabilities, fracture toughness, and other comprehensive properties. These superalloy are used in manufacturing of products such as aerospace vehicles, rocket engines, nuclear reactors,turbine blades, guide blades, turbine disks, high-pressure compressor disks,and combustion chambers for aviation, warship and industrial gas turbines.
Used In A Battery
Lithium-cobalt oxide (LiCoO2) is used to create the cathodes of lithium-ion batteries. Lithium is intercalated between the layers of cobalt oxides in these cathodes and to enhance the battery’s nickel oxidation it is also used in nickel-cadmium and nickel-metal hydride batteries. Due to its superior energy density and safety, lithium cobalt acid has emerged as the most used cathode material in lithium-ion batteries.
Used For Producing High End Magnet
Cobalt’s outer shell consists of unpaired electrons. Since they all spin in the same direction, the element is strongly magnetic. It has a Curie point of 1115 °C, which is substantially higher than that of iron and nickel. Above this point, materials start to lose their magnetic properties which makes cobalt suitable for use in high-temperature applications.
There are number of other various applications of cobalt including its use in color and pigmentations for art purpose, ceramics . They are also used as a catalyst and plays a vital role in electroplating. Other important use of this metal is done in jewellery industries and it is also used for producing prosthetics.
Health Effects Of Cobalt
- Cobalt is a trace mineral that the human body requires in minute quantities. A trace mineral is a tiny amount of an element that both plants and animals require. When trace metals are missing from a diet, it causes health concerns. Trace minerals are used by animals to create enzymes that act as catalysts. A key coenzyme of cell mitosis is cobalt. It is also essential in the formation of amino acids and proteins, which are important for the formation of myelin sheaths on nerve cells.
- The main component of vitamin B12, also known as cobalamin, is cobalt.Red blood cell production in the human body is ensured by B-12 vitamins. The majority of animals’ metabolisms depend heavily on it. Cobalt is converted into Vitamin B12 by microorganisms in the stomachs of ruminant animals.
- Various health issues can result from excessive cobalt. Workers who are exposed to cobalt dust may have nausea, constipation, or respiratory difficulties as a result. A rash and irritation can result from cobalt exposure to the skin.
Environmental Effects of Cobalt
- Small amounts of cobalt are emitted into the atmosphere by humans as a result of the combustion of coal, mineral extraction, the processing of cobalt-containing ores, and creating and using cobalt-containing chemicals.
- The radioactive isotopes of cobalt are not found in nature; rather, they are emitted during the operation of nuclear power plants and during nuclear accidents. They aren’t particularly harmful because of their limited half-lives. Once in the environment, cobalt cannot be destroyed. It might interact with different particles or stick to the water or soil. The majority of cobalt will eventually end up in soils and sediments, and it will only mobilize in acidic environments.
- Cobalt is quickly changing from a wonder metal to a lethal poison as toxic waste disposal destroys landscapes, contaminates water, and contaminates crops. Even the mortality of crops and worms, which are essential for soil fertility, has been connected to high cobalt concentrations.
- Fish in Congo’s Tshangalale Lake, that’s adjacent to mining towns, have been identified to contain high quantities of cobalt. Humans can readily get infected by this pollution by eating fish or drinking lake water. Being a radioactive element and being listed as a “possible” carcinogen, this also presents a serious risk to human health.
- The murky, polluting atmosphere surrounding the mines, which is full of sand and dust has another adverse impact of cobalt mining on the natural environment. According to studies, the high levels of hazardous contamination brought on by the extraction of cobalt increase the incidence of birth abnormalities, which include spina bifida, and limb deformities, significantly in families where one or both parents worked in a cobalt mine.
Interesting Facts About Cobalt
- Cobalt is named after mythical, death-dealing goblins.
- Cobalt can treat cancer, but it can potentially be fatal.
- Beers used to contain cobalt as an ingredient, which had devastating results.
- A Significant Quantum Physics Discovery Involved Cobalt.
- Food Safety May Be Improved by Cobalt.
- “Two-thirds of the world’s cobalt, a vital component in our smartphones and electric vehicles, comes from one of the world’s poorest countries.”
Watch out the video to learn some additional information related to Cobalt.
References
- Verhoeven, J.D. (1975) Fundamentals of Physical Metallurgy, Wiley, New York
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 978-0-7506-3365-9.
- Hampel, Clifford A. (1968). The Encyclopedia of the Chemical Elements. Van Nostrand Reinhold. ISBN 978-0-442-15598-8.
- https://www.lenntech.com/periodic/elements/co.htm
- https://geologyscience.com/ore-minerals/cobalt-ore/
- https://www.rsc.org/periodic-table/element/27/cobalt
- https://pubchem.ncbi.nlm.nih.gov/element/Cobalt#
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/3_d-Block_Elements/Group_09%3A_Transition_Metals/Chemistry_of_Cobalt
- Wang, Shijie (2006). “Cobalt—Its recovery, recycling, and application”. Journal of the Minerals, Metals and Materials Society.
- Sigel, Astrid; Sigel, Helmut; Sigel, Roland, eds. (2010). Organometallics in Environment and Toxicology (Metal Ions in Life Sciences). Cambridge, UK: Royal Society of Chemistry Publishing. ISBN 978-1-84755-177-1.

