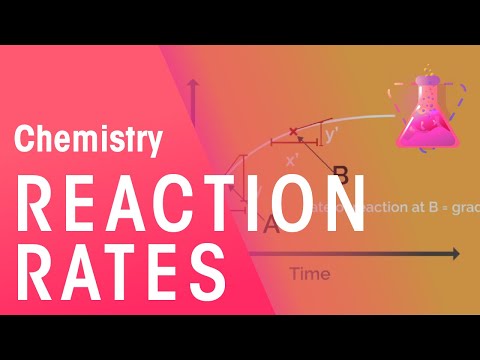
Chemical kinetics, also known as reaction kinetics, is the subfield of physical chemistry concerned with the study of the rates of chemical reactions. Reaction kinetics is the study of chemical reaction rates. The data obtained from rate experiments can shed light on how chemical reactions occur. Then, we can deduce the mechanism of a chemical reaction. Kinetic studies are important for understanding how reactions work and can be used in real life. Reaction kinetics investigation is inextricably linked to mechanisms and gives particular rate constants to various mechanistic stages.
What is Reaction Kinetics?
Reaction kinetics, often known as chemical kinetics, is a branch of chemistry that studies how different factors affect reaction speeds.
The study of the pace of chemical reactions, the factors that affect those rates, and the ways in which those studies may be applied to better understand reaction mechanisms is known as reaction kinetics, a subfield of chemistry.
Together, this aids in defining the properties of a chemical reaction and elucidating its underlying mechanism.
Rate experiments can help us understand how reactions happen by giving us information about how fast they happen. We can then draw conclusions about how a reaction works.
This is how you can describe the speed of a reaction:
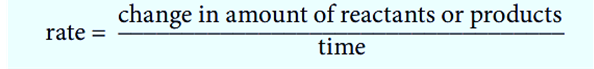
The balanced chemical equation doesn’t tell us anything about how fast a reaction is going. Experiments are needed to find out how fast reactants are used up or how fast products are made. For instance, if a reaction gives off a gas, we can measure the amount of gas given off at regular times during the reaction.
What is Rate of Reaction?
The speed at which reactants are changed into products is called the rate of reaction or reaction rate.
When we talk about chemical reactions, we know that the speed at which they happen can vary a lot. Some chemical reactions happen almost instantly, while others usually take a while to reach a state of balance.
In chemical systems, it is common to deal with the concentrations of substances, which is defined as the amount of substance per unit volume. The rate can then be thought of as the amount of a substance that is used up or made in a given amount of time. Sometimes it’s easier to talk about rates as the number of molecules made or used per unit of time.
History of Reaction Kinetics
The law of mass action, which was made by Peter Waage and Cato Guldberg in 1864, led to the field of chemical kinetics. The law of mass action says that the amount of reactants affects how fast a chemical reaction happens. Jacobus van’t Hoff looked into how chemicals move. The Nobel Prize in Chemistry was given to him in 1901 because of his 1884 book “Etudes de dynamique chimique” (which was the first year the Nobel prize was awarded).
Factors Affecting the Reaction Rate
Chemical kinetics indicates that variables that enhance the kinetic energy of the reactants (up to a degree) will increase the pace of the reaction, increasing the chance that the reactants will interact with each other. Similarly, conditions that reduce the likelihood of reactants interacting may be predicted to reduce the reaction rate. The following are the primary elements that influence response rate:
Nature of the reaction
The rate of reaction is heavily influenced by the kind and nature of the reaction.
The physical condition of the reactants, the quantity of reactants, the complexity of the reaction, and other factors all have a significant impact on the reaction rate.
The pace of reaction is often slower in liquids than in gases and slower in solids than in liquids. The size of the reactant is also important. The faster the reaction, the smaller the size of the reactant.
Effect of Concentration on Reaction Rate
According to the collision hypothesis, the rate of reaction rises as the concentration of the reactants increases. The chemical reaction rate is exactly proportional to the concentration of reactants, according to the law of mass action. This means that the chemical reaction rate increases as reactant concentration increases and reduces as reactant concentration falls. Similar to how concentration in solutions affects reactions involving gases, pressure has an impact on the same reactions. There are more gas molecules in a given volume as the pressure of reacting gases is raised. This causes a higher number of collisions every instant and a higher pace of reaction.
Time
Time has a significant impact on the concentration of reactants and products. As a result, time is a critical component influencing reaction pace.
Pressure
The concentration of gases increases as pressure increases, which increases the pace of reaction. The reaction rate accelerates in the direction of fewer gaseous molecules and slows in the opposite direction. As a result, it is clear that pressure and concentration are related and that they both influence the pace of response.
Temperature
A chemical reaction at a greater temperature creates more energy than a reaction at a lower temperature, according to collision theory. This is because colliding particles will have the needed activation energy at high temperatures, resulting in more successful collisions. There are several reactions that are temperature independent. Chemical reactions that do not have an activation barrier are examples of temperature-independent reactions.
At any given temperature, there will be energy differences among the particles in a sample of any substance. There will be a very modest quantity of energy in a few particles. The energy of a few particles will be comparatively high. The majority of particles will have an energy level that falls between the two. In a graph, the distribution of energy at a specific temperature can be displayed. This is known as the Boltzmann distribution.
Solvent
The kind of solvent also influences the pace of reaction. The reaction rate is heavily influenced by solvent properties and ionic strength.
Order
The sequence of reaction governs how reactant pressure or concentration impacts reaction pace.
Electromagnetic Radiation
Electromagnetic radiation is a kind of energy, and its presence in a chemical process may accelerate the pace of reaction by giving reactant particles more energy.
Light Intensity
The rate of response is affected by the intensity of the light. Particles absorb more energy as light intensity increases, boosting the pace of the reaction.
Catalyst
A catalyst is a material that accelerates the pace of a reaction without actually taking part in the process. Its influence on chemical processes is described in the definition.
The presence of a catalyst accelerates both forward and reverse reactions by offering an additional route with lower activation energy.
The Surface Area of Reactants
The rate of reaction is affected by the surface area of the reactants. The surface area of a particle rises as its size decreases, increasing the speed of heterogeneous chemical processes.
Collision Theory
According to collision theory, particles need to collide with enough energy and in the proper direction for them to interact.
The collision hypothesis is used to describe how concentration, temperature, surface area, and catalysts affect reaction speeds. The particles might be molecules, ions, or atoms. The reactant particles may just bounce off one another when they collide, remaining unchanged. This is called an unsuccessful collision. If the interacting particles lack the energy to respond, the collision will not succeed.
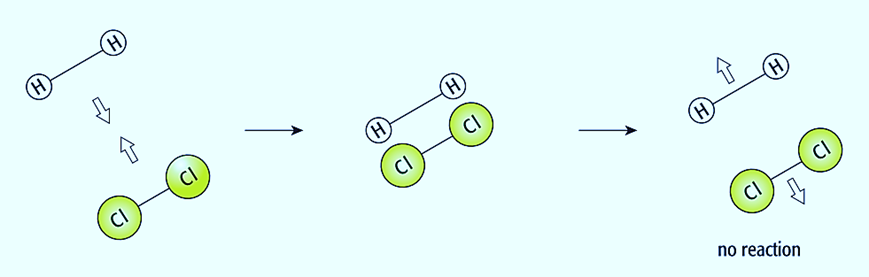
If the reactant particles have sufficient energy to react, when they collide, they could transform into product particles. This is referred to as a successful (or effective) collision. The activation energy of a certain reaction is the minimal amount of energy that two colliding particles need to possess to collide successfully.
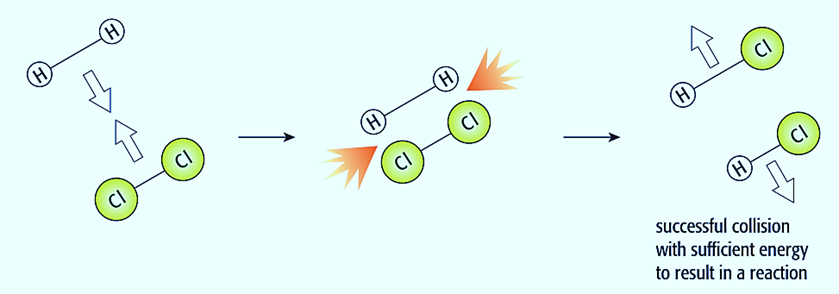
The collision hypothesis states that a reaction will accelerate if:
- collisions occur more frequently; and
- a higher fraction of particles have energy than the activation energy.
A catalyst is a material that speeds up a reaction while maintaining its own chemical integrity throughout the process. By enabling the particles to react through a different method, a catalyst does this. Lower activation energy is included in this substitute mechanism.
How does concentration affect the rate of reaction?
In chemistry, the standard unit of concentration is moles per decimeter cube, or moldm-3. The number of solute particles dissolved in a given volume of the solvent increases as a solution becomes more concentrated.
Greater concentrations of the reactants speed up the reaction in reactions involving solutions. This occurs as a result of more frequent collisions between responding particles as a result of the particles’ random mobility in solution. Similar to how concentration in solutions affects reactions involving gases, pressure has a similar impact. There are more gas molecules in a given volume when we increase the pressure of reacting gases. More collisions occur at any given period as a result, and the pace of reaction is accelerated.
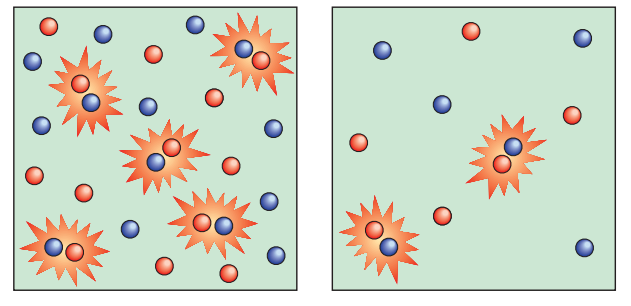
Similar to the impact of concentration in solutions, pressure has an impact on gaseous processes. More gas molecules can fit into a given volume when we increase the pressure of reacting gases. As a result, there are more collisions at any one time and the rate of reaction is accelerated.
How temperature affects the rate of a reaction?
We must pay more attention to the energy held by the reactant particles if we are to properly comprehend reaction rates.
At a specific temperature, the particles in a sample of any material won’t all have the same amount of energy. The energy of a few particles will be comparatively low. There will be a few particles with a significant amount of energy. Most particles will have an energy level that falls somewhere in the middle. A graph can display the distribution of energy at a specific temperature. The Boltzmann distribution causes this.
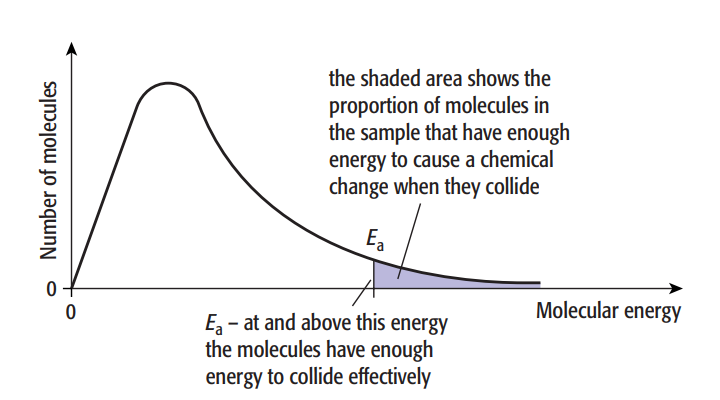
As we have seen, activation energy is the minimal amount of energy needed for particles to collide to produce a reaction.
The average kinetic (movement) energy of the particles in a reaction mixture rises as the temperature of the mixture rises. At a greater temperature, particles in gases and solutions will move more quickly, increasing the likelihood of collisions. Experiments, however, demonstrate that the influence of temperature on response rate cannot entirely be accounted for by more frequent collisions.
The crucial aspect is that when we raise the temperature, the fraction of successful collisions rises sharply. At higher temperatures, the Boltzmann distribution curve flattens and the peak moves to the right.
The number of particles is shown by the area under the curve. The amount of particles with energy larger than the activation energy, Ea, is indicated by the shaded region. This area under the curve about doubles with a 10 °C increase in temperature, as does the rate of many processes.
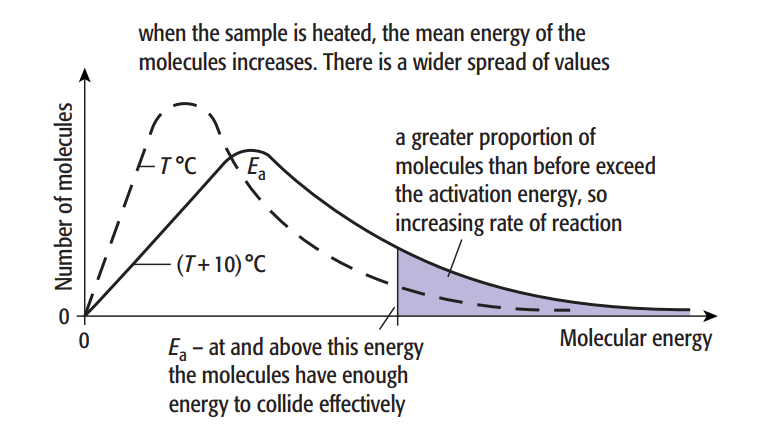
energies at temperatures T °C and (T + 10)°C
As a result, raising the temperature accelerates the reaction because: the increased energy causes the particles to move more quickly, increasing the likelihood of collisions.
Since more particles have energies greater than the activation energy, more successful collisions, or collisions that trigger a reaction, occur.
Watch and learn what the reaction rate is and some ways of measuring reaction rate.
References
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms (2nd ed., McGraw-Hill 2002), ISBN 0-07-288362-6
- Atkins P. and de Paula J., Physical Chemistry (8th ed., W.H. Freeman 2006) ISBN 0-7167-8759-8
- Steinfeld J.I., Francisco J.S. and Hase W.L. Chemical Kinetics and Dynamics (2nd ed., Prentice-Hall 1999) ISBN 0-13-737123-3
- Laidler, K.J. Chemical Kinetics (3rd ed., Harper and Row 1987) ISBN 0-06-043862-2
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms (2nd ed., McGraw-Hill 2002), ISBN 0-07-288362-6