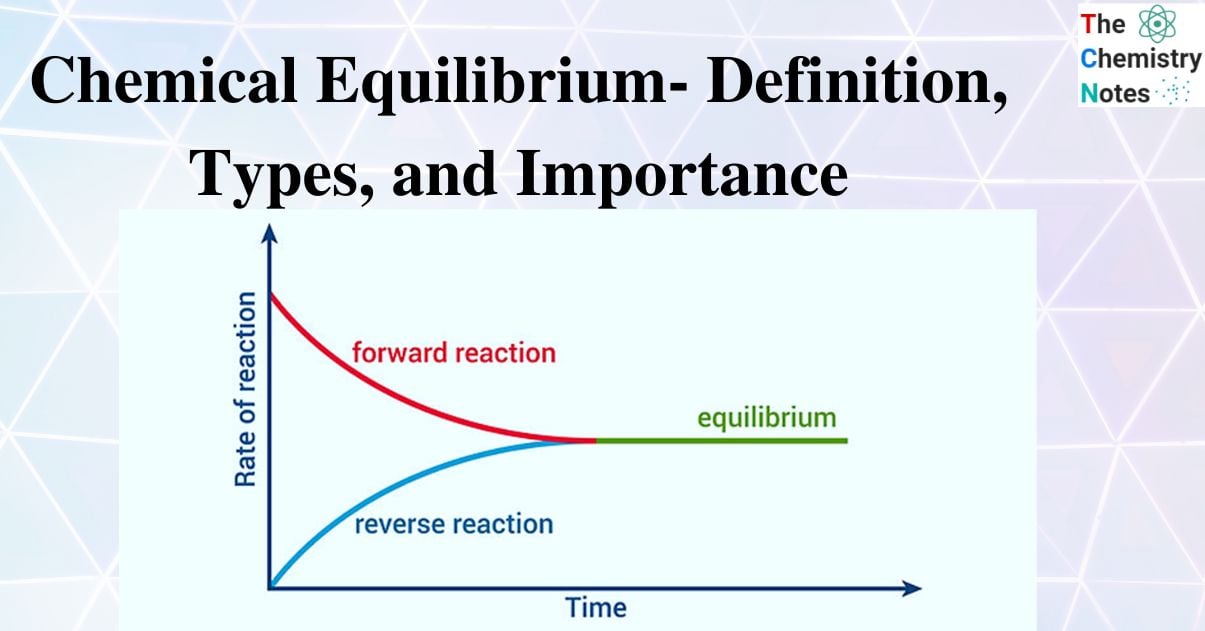
Chemical equilibrium is defined as the state of a system in which the concentration of the reactant and the concentration of the products do not fluctuate over time and the system exhibits no additional change in properties. When the rate of the forward reaction equals the rate of the reverse reaction, the system is said to have reached chemical equilibrium. The system is considered to be in a dynamic state of equilibrium when the concentrations of the reactants and products do not change further as a result of the equal rates of the forward and reverse reactions at that moment.
What is Chemical Equilibrium?
Chemistry defines chemical equilibrium as the dynamic state of the system where the concentration of reactants and products no longer exhibits a tendency to change over time. At the equilibrium point, there is no change in the relative rates or velocities of the backward and forward reactions.
Most chemical reactions fail to complete even when favorable external circumstances such as temperature, pressure, chemical catalyst, and pH scale of reaction are maintained.
In 1867, Norwegian physicists Guldberg and Waage established the dynamic link between the equilibrium constant and the concentrations of reactants and products at the equilibrium. It is known as the Law of Mass Action.
Characteristics of equilibrium
An equilibrium reaction has four particular features under constant conditions:
- Equilibrium is a dynamic process. Even though we do not visualize the reactions, both forward and reverse are taking place.
- The rates of the forward and reverse reactions must be equal.
- The amount of reactants and products do not have to be equal. However, after equilibrium is attained, the amounts of reactants and products will be constant.
- A close system, meaning no substances can enter or leave the system.
It is dynamic: The phrase dynamic equilibrium means that the molecules or ions of reactants and products are continuously reacting. Reactants are continuously changing to products and products are continuously changing back to reactants.
The forward and reverse reactions occur at the same rate : At equilibrium the rate of the forward reaction equals the rate of the backward reaction. Molecules or ions of reactants are becoming products, and those in the products are becoming reactants, at the same rate.
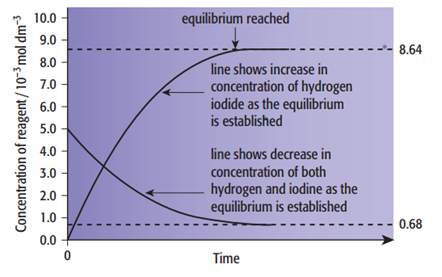
The concentrations of reactants and products remain constant at equilibrium: The concentrations remain constant because, at equilibrium, the rates of the forward and backward reactions are equal. The equilibrium approaches from two directions. For example, in the reaction
H2 (g) + I2 (g) → 2HI (g)
It requires a closed system: A closed system is one in which none of the reactants or products escapes from the reaction mixture. In an open system, there exists some loss of matter in the surroundings. Many chemical reactions can be studied without placing them in closed containers. In open flasks, they can come to equilibrium if the reaction occurs totally in solution with no gas loss.
CaCO3 (s) ⇌ CaO (s) + CO2 (g)
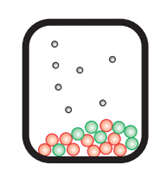
In a closed system: No carbon dioxide escapes. The calcium carbonate is in equilibrium with calcium oxide and carbon dioxide.
CaCO3 (s) → CaO (s) + CO2 (g)
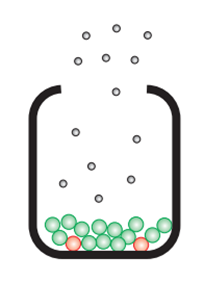
In an open system: The calcium carbonate is continually decomposing due to the loss of the carbon dioxide. The reaction eventually goes to completion.
Types of Chemical Equilibrium
There are two types of chemical equilibrium. They are:
- Homogeneous Equilibrium
- Heterogeneous Equilibrium
Homogeneous Equilibrium
In a homogeneous equilibrium, all of the reacting components are in the same phase or state of matter, such as solid, liquid, or gas.
These fall under three categories.
- The reaction in which there was no net change in the system’s mole numbers (Δγ = 0).
- The system’s mole numbers rise as a result of the reaction (Δγ = +ve).
- The system’s mole numbers decrease due to the reaction (Δγ = ve).
Heterogeneous Equilibrium
In a heterogeneous equilibrium, all of the reactive components are not in the same phase. Consider the breakdown of calcium carbonate into calcium oxide and carbon dioxide.
The equation represented,
CaCO3 (s) ⇌ CaO (s) + CO2 (g)
Changing the position of equilibrium
Position of equilibrium
The position of equilibrium refers to the relative amounts of products and reactants present in an equilibrium mixture.
- Disturbing the system in equilibrium (e.g. by a change in temperature) and increasing the concentration of products relative to the reactants, we say that the position of equilibrium has shifted to the right.
- Decreasing the concentration of products relative to the reactants, we say that the position of equilibrium has shifted to the left.
Factors Affecting Chemical Equilibrium
According to Le-principle, Chatelier’s if any of the components affecting the equilibrium circumstances change, the system will counteract or diminish the overall transformation’s effect. Equilibrium in both chemical and physical systems is governed by this idea. Equilibrium conditions are influenced by a number of variables, including system concentration, temperature, and pressure.
Change in Pressure
The change in volume causes the change in pressure. When the pressure changes, the total quantity of gaseous reactants and products changes, which might impact the gaseous reaction. Le Chatelier’s principle states that in the heterogeneous chemical equilibrium, a change in pressure in both liquids and solids can be neglected because the volume is independent of the pressure.
Variation in Temperature
By Le-Principle, Chatelier’s the influence of temperature on chemical equilibrium is dependent on the sign of ΔH of the reaction. An exothermic reaction equilibrium constant lowers as temperature rises. When an endothermic reaction occurs, the equilibrium constant rises as the temperature rises.
The change in temperature has an impact on both the equilibrium constant and the rate of response. Le Chatelier’s principle states that in exothermic processes, as temperature rises, the equilibrium moves toward the reactant side. With an increase in temperature, the equilibrium for endothermic processes changes toward the product side.
Effect of a Catalyst
A catalyst has no impact on the chemical balance. It just accelerates a reaction. In an equilibrium catalyzed reaction, the same number of reactants and products will be present as in an equilibrium non-catalyzed reaction. Only when the reaction passes through a lower-energy transition state of reactants to products does the presence of a catalyst aid.
Adding an Inert Gas and Its Effects
A consistent volume of an inert gas, such as argon, is introduced without causing the equilibrium to change because it does not participate in the reaction. Depending on whether the gas is a reactant or a product in the process, the reaction quotient will change.
Examples of Chemical equilibrium
In chemical reactions, the forward reaction transforms the reactants into the products, and the backward reaction transforms the products back into the reactants. Reactants and products exist in two different states and in many mixtures.
The pace of the forward and backward reactions may eventually become equal once the reaction has begun. After that, the reverse reaction will again produce the same number of reactants that gets transform, preventing any further changes in the concentration of reactants and products. Therefore, chemical equilibrium will exist between the reactants and products.
N2O4 ⇌ 2NO2
PCl5 ⇌ PCl3 +PCl2
N2 + H2 ⇌ 2NH3
Importance of Chemical Equilibrium
It is helpful in numerous industrial operations, including;
- Preparation of lime from limestone
- Production of methanol
- Ammonia preparation using Haber’s process: In this process, nitrogen mixes with hydrogen to generate ammonia; the yield of ammonia is higher at low temperatures, high pressures, and in the presence of iron as a catalyst.
- Sulfuric acid preparation via contacts process: The primary reaction in this process is the oxidation of sulfur dioxide into sulfur trioxide. This involves equilibrium in chemistry.
References
- P. W. Atkins, Physical Chemistry, 7th Ed.(2002) Oxford University Press, New York.
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Chemistry_for_Allied_Health_(Soult)/08%3A_Properties_of_Solutions/8.02%3A_Chemical_Equilibrium
- https://www.priyamstudycentre.com/2019/03/chemical-equilibrium-reaction.html
- https://www.britannica.com/science/chemical-equilibrium
- https://www.vedantu.com/chemistry/chemical-equilibrium
- https://ecampusontario.pressbooks.pub/queenschem1/chapter/chapter-12-chemical-equilibrium/
