Chemical bonding is the attractive force that holds different components (atoms, ions, etc.) together and stabilizes them via the total loss of energy. It is one of the most fundamental principles of chemistry, which helps to explain other ideas like molecules and reactions.
One must first understand the fundamentals of atomic structure in order to comprehend the concept of bonding. The attraction between two or more atoms that enables them to combine to produce a stable chemical compound is known as chemical bonding.
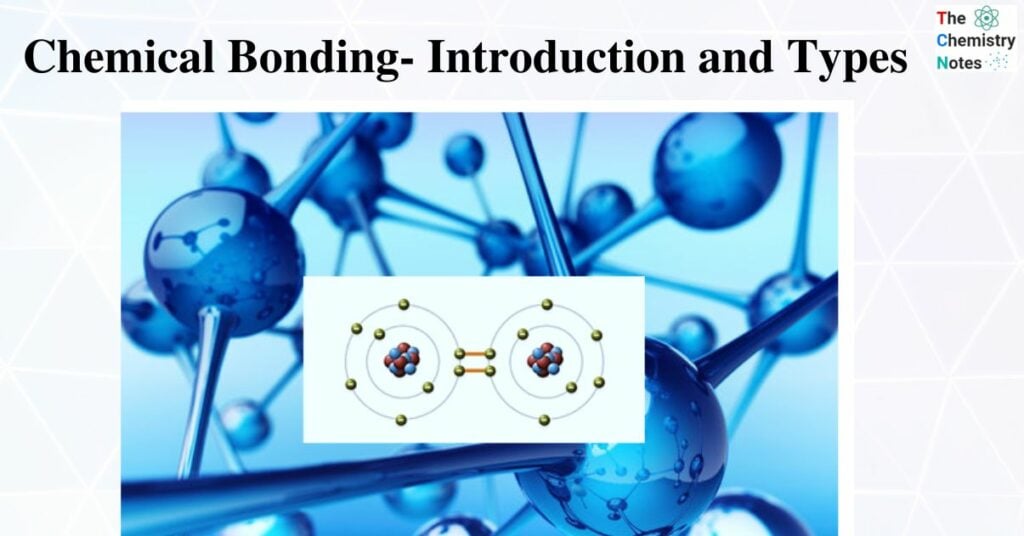
Therefore, it is clear that chemical compounds depend on the strength of the chemical bonds between their constituents; the more stable the resulting molecule, the stronger the bonding between the constituents. Likewise; if the constituents’ chemical bonds are weak, the resulting product will lack stability and will readily undergo another reaction to produce a more stable chemical complex (containing stronger bonds). The atoms struggle to release their energy in order to find stability.
Types of chemical bonding
When atomic building blocks are sufficiently close to one another, their outside (valence) electrons are drawn to the positive nuclear charge of their neighbor, forming a molecular bond. Both repulsive and attractive forces are present as the free atoms move closer to one another. To overcome the initial repulsive forces, some constituents require the input of energy, known as activation energy. However, at varying distances, the atoms experience distinct attractive and repulsive forces, eventually determining the ideal separation distance where the electrostatic forces are minimized.
The strength and characteristics of the many chemical bonds produced differ. Atoms or molecules join together to form compounds through the formation of 4 different types of chemical bonds. These specific chemical bonds consist of:
- Ionic Bonding
- Covalent Bonding
- Metallic Bonding
The electrostatic interaction between positive and negative ions in an ionic crystal lattice is known as ionic bonding.
When the outer electrons of two atoms are shared, covalent bonds are created. The ionic or covalent connections that are created are often quite powerful and need a lot of energy to break. Metallic bonding is the third type of strong bonding. Although molecules are held together by weak forces called covalent bonds, the atoms within them are held together by strong covalent bonds. These weak forces are referred to as intermolecular forces.
There are several types of intermolecular force:
- van der Waals’ forces (also called ‘dispersion forces’ and ‘temporary dipole–induced dipole forces’)
- permanent dipole–dipole forces
- hydrogen bonds
We can explain the structure and physical properties of elements and compounds by using knowledge of these many kinds of chemical bonding and intermolecular forces.
Ionic bonding
The strong attraction between the positive and negative ions in the ionic crystal lattice results in an ionic bond. An ionic bond is also known as an electrovalent bond. The ions are set up in an orderly, repeating pattern in an ionic framework. As a result, the force between one ion and the ions of opposite charge that surround it is extremely strong. In other words, ionic bonding is quite strong.
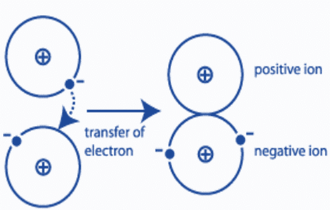
- Atoms can acquire or lose one or more electrons, which is one way that ions can form.
- When an atom loses one or more electrons, positive ions are created. Normally, metal atoms lose electrons and produce positive ions.
- The charge on the ion depends on the number of electrons lost or gained.
- When metals combine with non-metals, the electrons in the outer shell of the metal atoms are transferred to the non-metal atoms. Each non-metal atom usually gains enough electrons to fill its outer shell. As a result of this, the metal and non-metal atoms usually end up with outer electron shells that are complete – they have an electronic configuration of a noble gas.
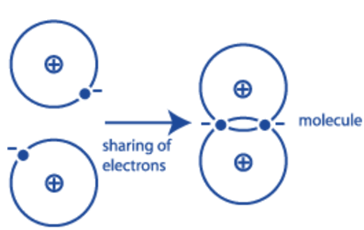
Covalent bonding
The process of sharing electrons between two atoms is known as covalent bonding. The bonds often exist between two nonmetals.
The electrons are drawn and attracted by the nuclei of both atoms because their electronegativities are all in the high range. Two identical atoms that are bound to one another (also known as a nonpolar bond) emit the same force on their electrons, which results in an equal attraction between the two atoms (for example, oxygen gas, or O2, has an equally distributed electron affinity). Thus, covalent connections are more difficult to break.
Single covalent bonds
When two non-metal atoms combine, they share one, or more, pairs of electrons. A single covalent bond, also known as a bond pair, is an electron pair that is shared.
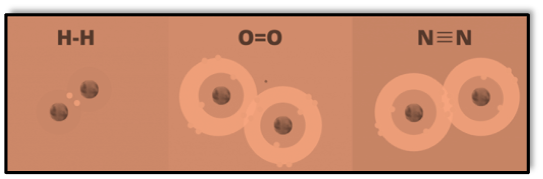
A single line between the atoms, such as Cl – Cl, represents a single covalent link.
As you can see, not every available electron is utilized to form bonds when chlorine atoms come together. Lone pairs are the outer-shell electron pairs that are not involved in forming bonds. Each chlorine atom possesses three lone pairs of electrons and shares one pair of electrons for a bond.
When drawing the arrangement of electrons in a molecule
- Use a “dot” to represent an electron from one atom and a “cross” to represent an electron from another atom.
- If there are more than two types of atoms, additional symbols, like a small circle or a small triangle, may be used.
- To emphasize the number of bond pairs and the number of lone pairs, we draw the outer electrons in pairs.
For example, Boron trifluoride BF3 has only six electrons around the boron atom; we say that the boron atom is electron deficient.
Sulfur hexafluoride, SF6, has twelve electrons around the central sulfur atom; we say that the sulfur atom has an ‘expanded octet’.
Multiple covalent bonds
Some atoms can form bonds by sharing two electron pairs. It is referred to as a double covalent bond.
A double line drawn between the atoms signifies a double covalent link, such as O=O. In order to form an oxygen molecule, each oxygen atom needs to gain two electrons to complete its outer shell. So, two pairs of electrons are shared and two covalent bonds are formed.
- Each oxygen atom must gain two electrons once more in order to produce carbon dioxide. However, the carbon atom still requires four additional electrons to finish off its outer shell. As a result, the carbon atom has eight electrons surrounding it since two oxygen atoms make each two bonds with carbon.
- Each carbon atom in ethene shares a pair of electrons with two hydrogen atoms. Each carbon atom now has two outer shell electrons available for intermolecular bonds. A double bond develops.
Atoms can also bond together by sharing three pairs of electrons. We call this a triple covalent bond.
In order to form a nitrogen molecule, each nitrogen atom needs to gain three electrons to complete its outer shell. So, three pairs of electrons are shared and three covalent bonds are formed.
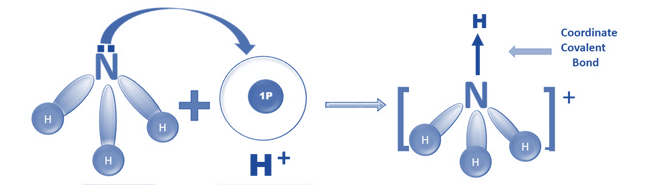
Co-ordinate bonding (dative covalent bonding)
A coordinate bond (or dative covalent bond) is formed when one atom provides both the electrons needed for a covalent bond.
We require an electron-deficient compound, which consists of two atoms:
- one with a lone pair of electrons; and
- the other with an unfilled orbital to accept the lone pair, for dative covalent bonding.
An example of this is the ammonium ion, NH4+, formed when ammonia combines with a hydrogen ion, H+. With two electrons available in its shell, the hydrogen ion is electron-deficient. One electron and one hole are present on the nitrogen atom of the ammonia molecule. Both of the bond’s electrons come from the nitrogen atom’s lone pair.
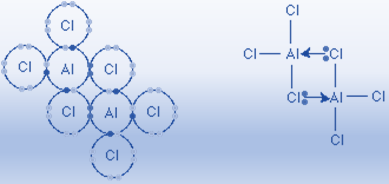
Aluminum chloride is a similar molecule that possesses coordinate bonds. Aluminum chloride exists at high temperatures as molecules having the formula AlCl3. In order to complete the aluminum atom’s outer shell, this molecule is electron-deficient and still requires two electrons. At lower temperatures, two AlCl3 molecules unite to generate an Al2Cl6 molecule. Lone pairs of electrons on two chlorine atoms create coordinate bonds with the aluminum atoms, allowing the AlCl3 molecules to unite.
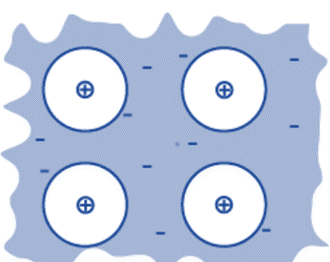
Metallic Bonding
The atoms of a metal are tightly packed together in a pattern known as a lattice. In a lattice, metal atoms frequently lose their outer shell electrons and transform them into positive ions. The electrons in the outer shell are free to move about the metal lattice and occupy new energy levels. These are what we refer to as delocalized electrons (mobile electrons). Electrons that are delocalized are those that are not connected to any one specific atom or bond. Strong metallic bonding exists. Because of the strong electrostatic attraction between the ions’ positive charges and the delocalized electrons’ negative charges, the ions are kept together. This electrostatic attraction acts in all directions.
The strength of metallic bonding increases with:
- increasing positive charge on the ions in the metal lattice
- decreasing the size of metal ions in the lattice
- an increasing number of mobile electrons per atom.
Although we have only covered ionic, covalent, and metallic bonding, there are numerous more atom-to-atom interactions and connections that can exist, most notably intermolecular forces (the interactions that exist between, rather than within, covalently bonded molecules).
References
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Chemical_Compounds/Introduction_to_Chemical_ Bonding
- https://byjus.com/jee/chemicalbonding
- https://www.britannica.com/science/ chemical-bonding
- https://www.visionlearning.com/en/library/Chemistry/1/ Chemical-Bonding/55
- Pauling, L. (1931), “The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules”, Journal of the American Chemical Society, 53 (4): 1367–1400, doi:10.1021/ja01355a027
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- Joseph J. Stephanos, Anthony W. Addison, in Electrons, Atoms, and Molecules in Inorganic Chemistry, 2017
