We use and are surrounded by batteries on a daily basis Whether it be in your phone’s batteries, the TV remote, video games, AC remotes, or car batteries. Any battery or cell that we use as a source of voltage is essentially a cell where the energy of the redox response or the reaction is converted into voltage.
Interesting Science Videos
Battery
A battery is a device that transforms chemical energy into electrical energy. It can have one or more electrical cells. In essence, every battery is a galvanic cell that generates chemical energy through redox reactions between two electrodes. An electrochemical cell, or series of electrochemical cells, that generates an electric current, is a battery.
By combining two different electrodes, you can create a galvanic cell. All of the galvanic cells, however, cannot be used as useful cells or batteries. Typically, a collection of a few similar-natured cells is referred to as a battery. The standard dry cells found in radios, toys, and torches have 1.5 to 2.0 volts of voltage. To generate enough power, several of these cells are frequently required, and the voltage of these so-called “batteries” gradually decreases over time.
In recent years, a wide range of electrochemical cells has been created for various purposes. Although many cells are small, they do not always produce a high voltage for a prolonged period of time. Higher voltage is provided by batteries made of multiple cells connected together, but they are larger. The following factors must be taken into account when choosing a cell for a particular task: the cell’s ability to be recharged; its size and mass; its voltage; the composition of its electrolyte; the duration for which the cell can deliver its maximum voltage; and the cost of the cell.
The following qualities are necessary for a battery to function properly:
- It must be small and light in weight.
- The cell or battery must deliver a constant voltage. Additionally, the voltage of the battery or cell cannot change while it is being used.
Types of Battery
There are primarily two types of batteries or useful cells used in commerce. They are:
- primary battery or cell
- secondary battery or cell.
Primary Cells
The primary batteries are non-rechargeable and only intended for single use. These kinds of batteries cannot be recharged after they have been used because the devices are not easily reversible and the active materials might not revert to their original forms.
These disposable batteries include the typical AA and AAA batteries used in wall clocks, television remotes, and other electrical devices. The electricity is generated by the primary cells as a result of a chemical reaction. In this case, there is only one direction of the reaction. Thus we are unable to stop this phenomenon. As a result, over time, these cells start to die.
For Example: Daniell cell, Dry cell, and Mercury cell are a few types of primary cells.
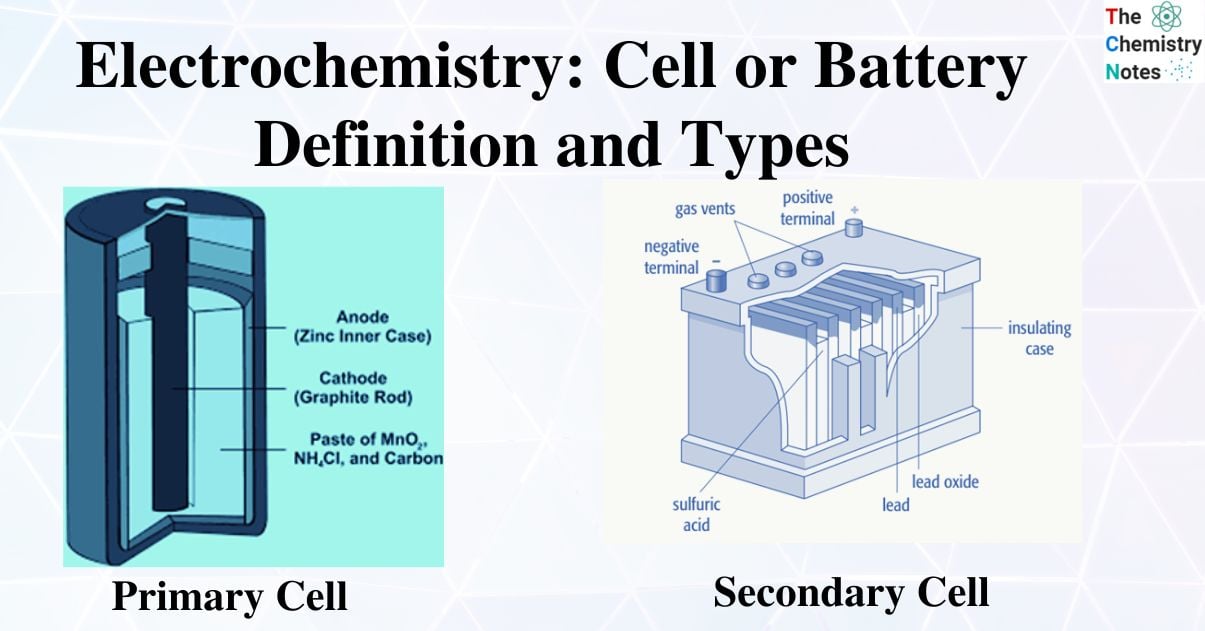
Dry Cell
The dry cell is a more compact variation of the LeClanche cell. It consists of an inc outer container that serves as an anode. A porous insulating paper lines the interior of the zinc-containing cell. A carbon rod with a brass cap serves as the cathode.
In the area between the cathode and the anode, there is a mixture of MnO2 and a viscous paste of charcoal, zinc chloride, and ammonium chloride (NH4Cl). The porous paper’s lining keeps the paste and zinc container from coming into direct contact. It serves as a bridge for salt. Pitch or wax is used to seal the cell from the top.
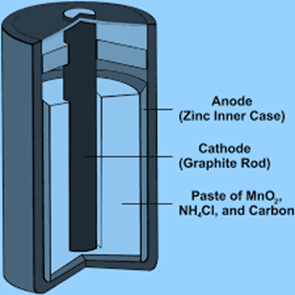
Reactions during discharge
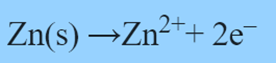
Zn2+ ions move toward the carbon electrode (cathode). The cathode’s reaction is as follows:

It has a depolarizing effect. In a cathodic reaction, manganese is changed from + 4 to + 3 states. Zn (NH3)42+ is created when the ammonia molecules created at the cathode interact with Zn2+ ions coming from the anode. The interaction of NH3 molecules with Zn2+ reduces the amount of free Zn2+ and raises the voltage of the cell. The potential of a dry cell is approximately 1.5 V.
Are dry cells actually dry?
The dry cells aren’t really dry, though. They have an NH4Cl and ZnCl2 wet paste. A dry cell will actually only work as long as the paste inside it is moist. Additionally, a dry cell cannot be recharged. Thus, it follows that the dry cells do not have eternal life. This is due to the acidic nature of the NH4Cl paste, which continues to corrode the zinc container even when it is not in use.
Alkaline Battery
Alkaline batteries were developed in the 1950s to address some of the performance issues with zinc-carbon dry cells. They are designed to be exact replicas of dry zinc-carbon cells. These batteries use alkaline electrolytes, such as potassium hydroxide.
Anode: Zn(s) + 2OH–(aq) → ZnO(s) + H2O(l) +2e–
Eº anode = −1.28V
Cathode: 2MnO2 (s) + H2O (l) + 2e– → Mn2O3 (s) + 2OH– (aq)
Eºcathode = + 0.15V
Overall: Zn (s) + 2MnO2(s) → ZnO (s) + Mn2O3(s)
Eºcell = +1.43V
Comparable-sized zinc-carbon dry cells can provide three to five times the energy of an alkaline battery. Alkaline batteries should be taken out of any devices before being stored for an extended period of time because they have a tendency to leak potassium hydroxide.
Mercury cell
It provides a somewhat more consistent voltage. The mercury cell’s emf is 1.35 V. The mercury cell typically costs more. They are only used in sophisticated instruments like cameras, hearing aids, watches, and other items because of this. In a mercury cell, the anode is a zinc alloy plate coated with a steel top plate.
The cathode is made of mercury, mercury oxide, and carbon powder paste. It comes into contact with the steel case’s exterior. The electrolyte is a paste of KOH and Zn(OH)2 that has been saturated. This paste is carried by a sterile porous substance. A neoprene rubber insulation seal keeps the two electrodes apart. The reactions upon discharge include,
At anode: Zn(Hg) + 2OH– →Zn (OH)2 + 2e–
At cathode: HgO + H2O + 2e– →Hg + 2OH–
Overall reaction: Zn(Hg) + HgO (s) →Zn(OH)2+ Hg (l)
Solid state cells

Primary cells have recently been created with increased voltage and decreased size. In addition to watches and calculators, cells the size of a large button are used in heart pacemakers, hearing aids, and other medical devices.
They offer a number of benefits:
- They are portable and small
- They provide a high voltage, such as 3.0V,
- Maintain a constant voltage over time,
- Do not contain liquids or paste, which prevents leakage.
Iodine, manganese (IV) oxide, or silver oxide are used as the positive poles in commonly used “button” cells, while lithium or zinc are used as the negative poles.
Secondary Battery
Rechargeable batteries are another name for secondary batteries. These batteries can be used and recharged at the same time.
On the other hand, repeated action cells are found in secondary cells. After each use, these cells can be recharged. The cells are recharged by electricity flowing through them. As a result, you can utilize these cells repeatedly. Lead-acid cells, also known as lead storage cells, nickel-cadmium cells, and others are a few examples of secondary cells.
- The active components in secondary batteries typically dissolve after use.
- Electric current is used to recharge rechargeable batteries, which turns around the chemical processes that take place during discharge.
- Electronic devices called chargers, provide the necessary current.
- Cell phones, MP3 players, and other electronic devices use rechargeable batteries.
- Compared to phone exchanges and computer data centers, which use larger batteries, hearing aids and wristwatches typically require small batteries.
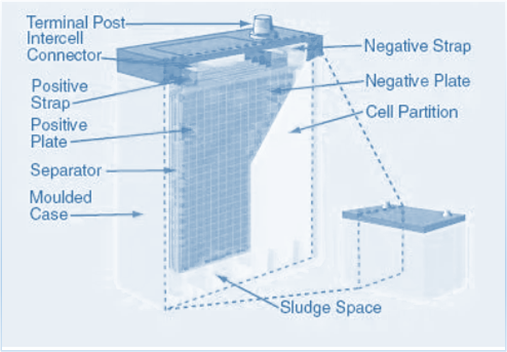
Lead – Acid Batteries
The most common and widely utilized rechargeable battery type is the lead-acid battery. Lead-acid batteries come in a variety of sizes, from tiny sealed cells with a capacity of 1 Ah to enormous sealed cells with a capacity of 12,000 Ah. These batteries are frequently used in the automotive sector, typically as SLI Batteries (Starting, Lighting, and Ignition). Lead-acid batteries can also be used for,
- Energy storage
- Backup power
- Electric vehicles (including hybrids)
- Communication systems
- Emergency lighting
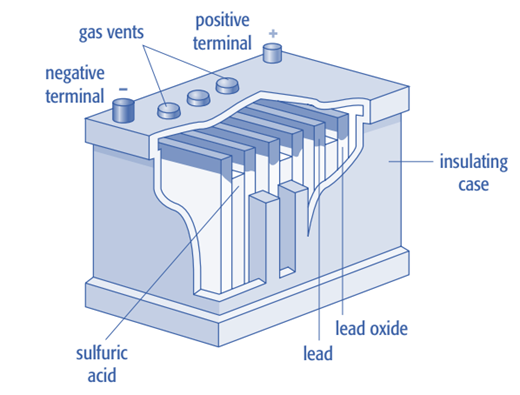
A car battery
A car battery is a secondary cell made up of lead and lead (IV) oxide plates that are submerged in sulfuric acid. Each cell has a voltage of 2V. A higher voltage is necessary to power the starter motor of the car. In order to provide 12V, six of these cells are connected in series to form a car battery. While the car engine is running, the alternator recharges the battery. Despite their heavy weight, lead-acid batteries are inexpensive to produce.
There have been advancements made to electric vehicle batteries.
- Nickel-cadmium batteries are lighter and smaller than lead-acid batteries, but they produce less voltage. Their “rundown” time is slower.
- Compared to lead-acid batteries, aluminum-air batteries are more portable and produce a higher voltage. They are costly and are not true secondary cells because of the frequent replacement of the aluminum anode.
Nickel – Cadmium Batteries
Nickel-Cadmium Batteries, or simply Ni-Cd Batteries, are some of the oldest batteries still in use today, along with lead-acid batteries. They are incredibly dependable and durable, and they have a long lifespan.
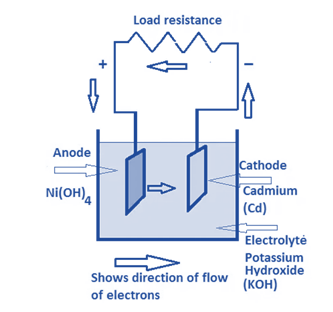
- One of the main benefits of Ni-Cd batteries is their ability to withstand high discharge rates and thus operate over a wide temperature range.
- Also having a very long shelf life are Ni-Cd batteries.
- These batteries are more expensive than lead-acid batteries per Watt-hour but less expensive than other alkaline battery varieties.
Nickel–Metal Hydride Batteries
These batteries are a more sophisticated version of the nickel-hydrogen electrode batteries that were previously only used in aerospace applications (satellites).
- The positive electrode is made of nickel oxide hydroxide (NiOOH), and the negative electrode is made of a metal alloy, where hydrogen is reversibly stored.
- One of the main advantages of nickel-metal hydride batteries over Ni-Cd batteries is that they have a higher specific energy and energy density.
- Commercially available small cylindrical sealed nickel-metal hydride batteries are mostly used in portable electronics.
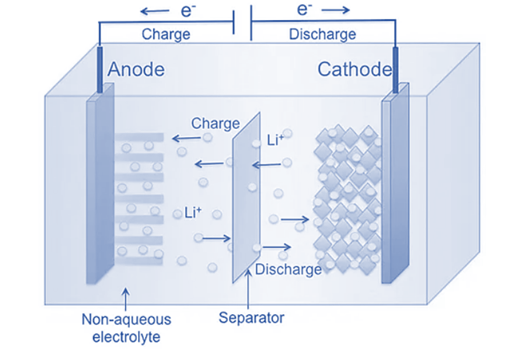
Lithium – Ion Batteries
Amazing progress has been made in lithium-ion battery technology over the past few decades.
More than half of the consumer market has embraced lithium-ion batteries.
Among the most popular applications for lithium-ion batteries are laptops, mobile phones, cameras, and other electronic devices.
Types of Battery Based on their Application
They can be classified further again based on their application. As follows:
Household Batteries
These are the battery types that the average person is more likely to be familiar with. They are used in a variety of home appliances (such as torches, clocks, and cameras). These batteries can be further divided into two additional groups:
Rechargeable batteries. Examples are Cadmium batteries, Lithium-Ion
Non-rechargeable batteries. Examples include carbon zinc, alkaline, and silver oxide.
Industrial Batteries
These batteries are designed to meet demanding needs. For large businesses, some of their applications include railroad, backup power, and more.
Examples include: Wet nickel, cadmium, and nickel-iron (NiCd)
Automobile Batteries
These are simpler, easier-to-use versions of industrial batteries. They are made especially to power cars, motorcycles, boats, and other types of vehicles. The lead-acid battery is hence a significant illustration of a car battery.
References
- https://collegedunia.com/exams/types-of-battery-primary-and-secondary-cell-and-their-uses-physics-articleid-1434
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_(Hill_and_McCreary)/08%3A_Oxidation_and_Reduction/8.03%3A_Electrochemistry-_Cells_and_Batteries
- https://classnotes.org.in/class12/chemistry12/electro-chemistry/batteries/#:~:text=A%20battery%20is%20an%20arrangement,is%20an%20oxidation%2Dreduction%20reaction.&text=1)%20It%20should%20be%20light,when%20it%20is%20not% 20used.
- https://unacademy.com/content/nda/study-material/chemistry/electrochemistry-battery_chemistry/
- https://www.toppr.com/guides/chemistry/electrochemistry/batteries/
- https://byjus.com/chemistry/battery-definition/
- https://www.edinformatics.com/math_science/how-does-a-battery-work.html
