Catalysis is a phenomenon in which the presence of a substance changes the rate of a chemical reaction without changing its qualitative and quantitative properties. For a long time, it has been known that the presence of minute amounts of certain compounds alters the rate of a chemical reaction. Such a substance was named a catalyst by Berzelius (18350), and the process was known as catalysis. A substance that, despite frequently being present in minute amounts, changes the rate of a chemical reaction while remaining chemically unaltered at the end is known as a catalyst.
A catalyst is an element or a chemical that lowers the activation energy of reactant molecules, allowing a greater number of molecules to collide at a high frequency. As a result of the effective collision, products are formed by overcoming the energy barrier imposed by the activated complex.A catalyst speeds up a chemical reaction by forming bonds with the reactant molecules. Generally, catalysts interact with one or more reactants to produce the intermediates that ultimately result in the final reaction product, hence regenerating the catalyst. Catalysts are not consumed during the chemical reaction and hence remain unchanged.
Interesting Science Videos
Examples of catalysis
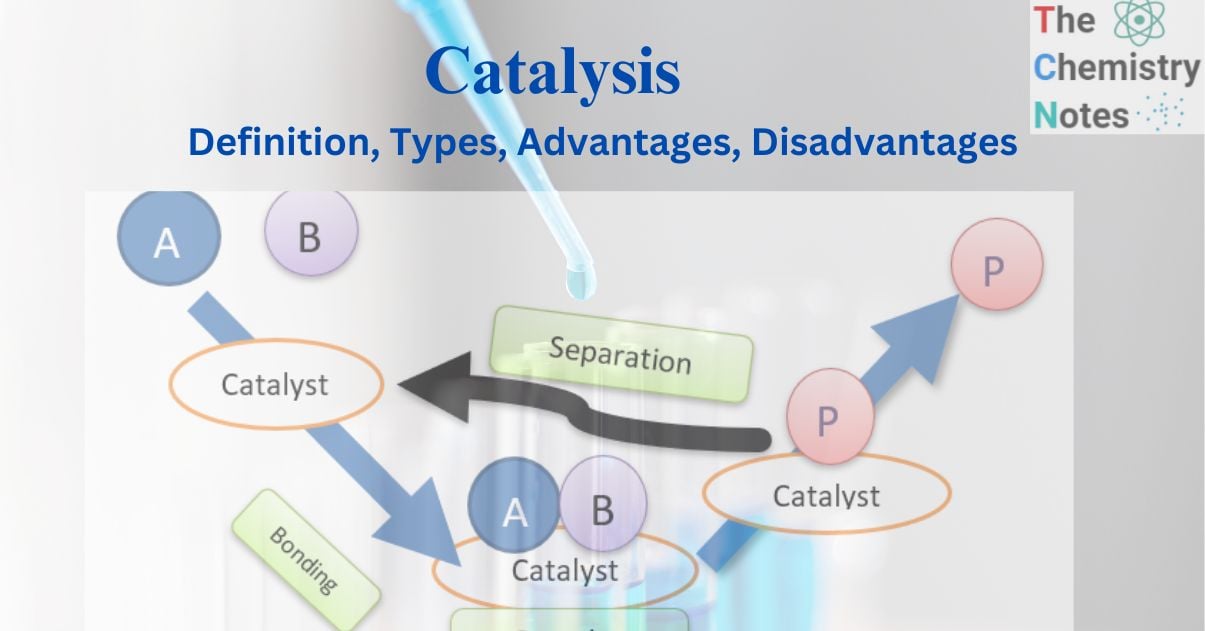
Consider the catalytic interaction between the molecules A and B, which results in the formation of the product P. The catalyst is initially bound to the molecules A and B to form the complex molecule. Thus formed complex produces product P, which is also bound to the catalyst. Ultimately, P separates from the catalyst, regenerating the catalyst.
i. If gases are passed over CuCl2, the oxidation of HCl occurs quickly.
4 HCl + O2 + CuCl2 (catalyst)→ 2H2O + 2 Cl2
ii. When Pt, a catalyst in this reaction, is present, the slow combination of H2 and O2 that produces H2O at ordinary temperature proceeds more quickly.
2 H2+ O2 + Pt (catalyst)→ 2H2O
Classification of catalysis
I. Classification based on the nature of Phase
Based on the nature of phase there are two different types of catalysis i.e.,
a. Homogeneous catalysis
b. Heterogeneous catalysis
Homogeneous catalysis
The catalyst is dispersed uniformly throughout the solution in homogeneous catalysis. In other words, the catalyst and reactions are in the same phase, and the entire reacting system is homogeneous. Homogeneous catalysts are often dissolved in a solvent. Examples of homogeneous catalysts are enzymes employed in biological reactions, mineral acids or alkalis used in the hydrolysis of esters, acid-catalyzed esterification, etc.
a. Reaction of SO2 and O2 in presence of No
2 SO2 (g) + O2 (g) + NO (g) (catalyst) → 2 SO3 + NO
b. Cane sugar hydrolysis in aqueous solution using mineral acid as a catalyst
C12H22O11 + H2O + H2SO4 (catalyst) → C6H12O6 (glucose)+ C6H12O6 (fructose)+ H2SO4
Heterogeneous catalysis
The catalyst and the reactants are in different phases in heterogeneous catalysis. The catalyst is usually solid, but the reactant can be either gaseous or liquid. Raney nickel, used in the hydrogenation of unsaturated organic compounds, and iron, utilized in ammonia synthesis, i.e. Haber’s method, are examples of heterogeneous catalysts.
a. Reaction of sulphur dioxide and oxygen in the presence of finely divided platinum and vanadium pentoxide (contact process for manufacture of sulphuric acid).
2 SO2 (g) + O2 (g) + Pt (s) (catalyst) → 2 SO3 + Pt
b. Haber’s process for manufacture of ammonia ; combination of nitrogen and hydrogen in presence of finely divided iron.
N2 (g) + 3 H2 (g) + Fe (s) (catalyst) → 2 NH3 + Fe
II. Catalysis based on the nature of catalyst
Based on the nature of catalyst, catalysis if further divided in to three different types they are:
a. Positive catalysis
b. Negative catalysis
c. Autocatalysis
d. Induced catalysis
Positive catalysis
When a catalyst increases the rate of chemical reaction, the phenomenon is known as positive catalysis. These catalysts are known as positive catalysts.
Some of the examples of positive catalysts are as follows:
- Enzymes used in biochemical reactions such as zymase.
- Inorganic acid or base used in organic hydrolysis reactions.
- Decomposition of KClO3 by heat in presence of MnO2.
2KClO3 + MnO2 → 2KCl+ 3O2
Negative catalysis
When a catalyst decreases the rate of chemical reaction, the phenomenon is known as negative catalysis. These catalysts are known as negative catalysts.
Some of the examples of positive catalysts are as follows:
a. The small amount of glycerol slows down the decomposition of H2O2.
b. Tetraethyl lead, or an antiknocking agent, is added to prevent the pre-ignition of oxygen and gasoline.
c. Adding a small amount of sugar, alcohol, or glycerol slows down the oxidation of sodium sulphite.
Induced catalysis
The phenomenon where one reaction causes another reaction is known as “induced catalysis.”
Sodium sulphite is quickly oxidized by air oxygen, but sodium arsenite is not often oxidized by the air. The oxidation of sodium sulphite stimulates the oxidation of sodium arsenite when air is introduced to a mixture containing both.
Na2SO3  Na2SO4
Na2SO4
Na2AsO3  No reaction
No reaction
Na2SO3+ Na2AsO3  Na2AsO4+ Na2SO4
Na2AsO4+ Na2SO4
Autocatalysis
A substance which is formed during the chemical reaction (not added externally) may act as a catalyst for the same reaction is called as autocatalyst and this phenomenon is known as autocatalysis.
For example,
a. When acidified potassium permanganate is introduced to warm oxalic acid, the permanganate does not decolorize immediately. However, once the first fraction of the permanganate is decolorized, the reaction becomes rapid. The manganese sulphate generated as a result of the reaction serves as an autocatalyst.
2KMnO4+ 5H2C2O4+ 6H+ → 2Mn2++ 10CO2+ 8H2O
b. The hydrolysis of ethyl acetate is catalyzed by acetic acid, which is generated throughout the reaction and functions as an autocatalyst.
Acid base catalysis: Lowery -Bronsted theory of acid-base catalysis
This explains any reactions that an acid or base catalyzes in an aqueous solution. This theory states that the proton transfer from the catalyst to the reactant X to form an intermediate complex XH, which then reacts by giving up the proton to another substance able to accept it.
a. Sulphuric acid serves as a catalyst in the production of ester from alcohol and acetic acid.
reaction mechanism:
C2H5OH + H+ → C2H5OH2+
C2H5OH2+ + CH3COOH → CH3COOC2H5 + H3O+
b. In the presence of an acid as a catalyst, the ester is hydrolyzed to the corresponding fatty acid and alcohol.
RCOOR + H2O + H3O+ ⇌ RCOOH (fatty acid) + ROH (alcohol)
Enzyme catalysis
Animals and live plants produce complex nitrogenous organic molecules called enzymes. These are protein molecules with a high molecular weight. They form colloidal solutions with water and are powerful catalysts. Their function is highly specific, i.e., only one enzyme catalyzes a single reaction. For example:
a. Maltase converts maltose into glucose.
C12H22O11 (maltose) + H2O → 2 C6H12O6 (in presence of maltase)
b. Enzyme, Zymase convertes the glucose to ethanol with liberation of carbon dioxide.
C6H12O6 (glucose) → 2 C2H5OH + CO2 (in presence of zymase)
Advantages of Homogeneous Catalyst over Heterogeneous Catalyst
Homogeneous catalysts have several advantages over heterogeneous catalysts as follows:
- Homogeneous catalysts have several advantages over heterogeneous catalysts, including: • A homogeneous catalyst is typically more selective and specific for a reaction than a heterogeneous catalyst.
- A homogeneous catalyst is simpler to characterize in the reaction mechanism.
Disadvantages of Homogeneous Catalyst over Heterogeneous Catalyst
- One main disadvantage of homogeneous catalysts is that they are difficult and costly to remove from the reaction mixture, whereas heterogeneous catalysts can be separated readily and cheaply.
- Homogeneous catalysts have low thermal stability, whereas heterogeneous catalysts have high thermal stability.
Characteristics of catalysts
Despite the considerable variations in type and chemical composition, the majority of catalysts share the following common characteristics:
i. Catalyst unchangeability
A catalyst remains chemically unaffected at the end of a chemical reaction. Though their chemical composition remains unchanged, their physical state may change. For example, After the end of the reaction, it is found that the granular manganese dioxide utilized in the thermal decomposition of potassium chlorate has converted into a fine powder.
ii. Small quantity
A small amount of catalyst is sufficient to achieve several reactions, provided the activity of the catalyst remains unaffected. This is because the catalyst is not consumed during the reaction.
iii. Do not alter the equilibrium point
In a system with two opposing reactions, a catalyst accelerates the reverse reaction to the same extent as the forward reaction, maintaining the ratio of their velocities and the equilibrium constant. In this way, the catalyst only quickens the rate at which the equilibrium point is reached rather than changing the equilibrium’s state.
iv. Catalyst can not start reaction
A catalyst cannot start a reaction. They just accelerate the reaction in progress.
v. Specificity
Only a specific type of reaction can be catalyzed by a specific catalyst. A chemical that acts as a catalyst in one reaction may not have any effect on another.
For example:
a. Nitric oxide is produced when ammonia is catalytically oxidized at 500 oC in the presence of platinum catalysts, and nitrogen is produced when copper catalysts are present.
4 NH3 + 5 O2 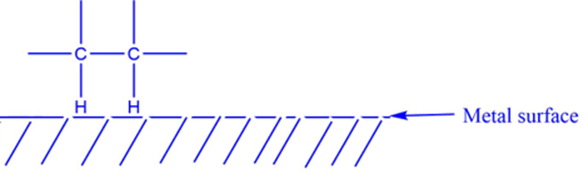 4 NO + 6H2O
4 NO + 6H2O
4 NH3 + 3 O2  2N2 + 6H2O
2N2 + 6H2O
b. At high temperatures and pressures, carbon monoxide and hydrogen react to produce methane and water in the presence of a nickel catalyst and methyl alcohol in the presence of a zinc oxide catalyst.
CO + 3 H2  CH4 + H2O
CH4 + H2O
CO + 2O2  CH3OH
CH3OH
Importance of catalysis
The catalytic procedure has a significant impact in many fields. Some of them are as follows:
In industry
Catalysts play a crucial role in various manufacturing activities, including the production of food and petroleum. They help in the manufacturing of chemicals and industrial refinement. Since catalyst control the activation energy needed to initiate chemical reactions, they make chemical production processes safer, simpler, and faster. Furthermore, catalysts are used in the manufacturing process of about 90% of all industrial chemicals produced worldwide. The following are some examples of catalysts used in industry:
- Iron: The Haber process uses iron to produce ammonia.
- Platinum – This metal is utilized in the Ostwald process, which produces nitric acid.
- Nickel- It is utilized in the production of vegetable ghee.
- Platinum and rhodium – These metals are employed in catalytic converters.
- Vanadium Oxide – This catalyst is utilized in the contact process to produce sulfuric acid.
In daily life
Enzymes are the biological catalysts that catalyze all of the metabolic processes that occur in our bodies and show the nature of the protein. A few nucleic acids also function as enzymes.
The following are some examples of biological enzyme-catalyzed reactions:
- Glucose to ethyl alcohol conversion- Zymase in yeast converts glucose to ethanol and carbon dioxide.
- Pepsin, an enzyme, breaks down proteins into peptides in the stomach.
- Rennin (an enzyme found in newborns) helps in the digestion of milk protein.
- Several enzymes found in pancreatic juice, such as trypsin and chymotrypsin, convert proteins into peptides.
- Nucleases are enzymes that convert nucleic acids into nucleotides.
- Lactobacillus produces lactic acid that converts milk into curd.
Environmental catalysis
In recent years, increasing environmental pollution has driven the rapid development of environmental catalysis. It helps in the catalytic conversion of biomass to biofuels. This also makes a significant contribution to sustainable development through the development of novel catalytic materials and technologies. It helps in the degradation of atmospheric pollutants, water, and soil remediation, hydrogen production, and carbon dioxide reduction.
Criteria for choosing catalyst for industrial applications
The following are some of the factors to consider while selecting a catalyst for various industrial applications:
- It must be stable under various reaction conditions.
- It should be affordable and widely available.
- It should be selective so that the desired product is the main product.
- It should have the highest degree of activity achievable for the particular chemical reaction under consideration.
Advantages of catalysis
The following are some of the advantages of using catalysts in chemical reactions:
- When a chemical reaction is performed in the presence of a catalyst, less energy is consumed.
- Since catalysts are not consumed in a reaction, they can be reused for other chemical reactions.
- It accelerates the reaction and reduces production expenses.
- A small amount of catalyst is sufficient to carry out a chemical reaction.
- It permits the reaction to occur at a significantly lower temperature.
Disadvantages of catalysis
In addition to the advantages of using catalysts in chemical reactions, there are a few disadvantages. The catalysis phenomenon has some disadvantages, including the following:
- Catalysts are quite expensive to purchase.
- As various reactions require different catalysts, multiple catalysts are required to produce more products.
- Impurities can cause catalysts to lose their effectiveness.
- They frequently need to be cleaned and removed from the products.
References
- https://www.britannica.com/science/catalysis.
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-The_Central_Science(Brown_et_al.)/14%3A_Chemical_Kinetics/14.07%3A_Catalysis.
- https://byjus.com/chemistry/catalysis/.
- https://application.wiley-vch.de/books/sample/3527316728_c01.pdf.
- https://www.uobabylon.edu.iq/eprints/publication_7_12100_563.pdf.
- https://www.lkouniv.ac.in/site/writereaddata/siteContent/202004070948263098nksingh_Catalysis.pdf.
- https://stannescet.ac.in/cms/staff/qbank/CSE/Notes/CY8151-Engineering%20Chemistry-431878289-unit_2%20(1).pdf.
