Calorimetry is the science of measuring the heat exchange between a system and its surroundings to calculate the change in energy of the system. The word Calorimetry is derived from the Latin calor (“heat”) and Greek metron (“measure”), and so means “measuring heat.”
What is Calorimetry?
Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.
To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. It is possible to tell whether a reaction is exothermic (releases heat) or endothermic by knowing the change in heat (absorbs heat). Calorimetry also has a significant impact on daily living since it regulates human metabolic rates, which helps to keep things like body temperature stable.
A calorimeter is used to perform calorimetry.
Principle of Calorimetry
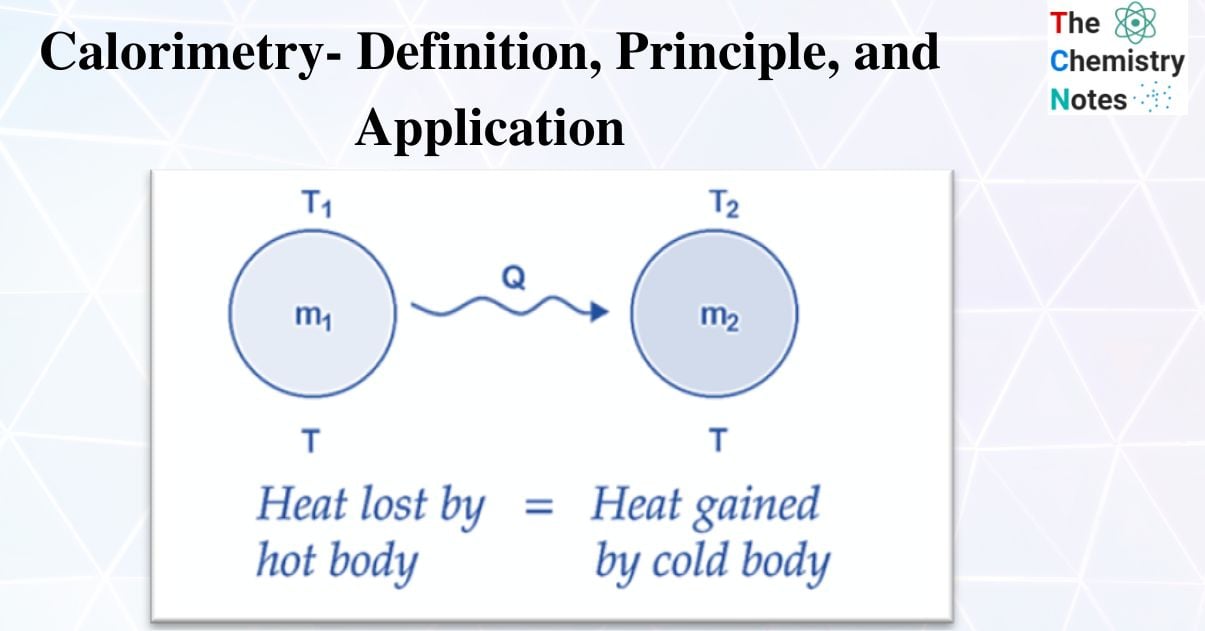
When a hot body and a cold body are combined, the heat lost by the hot body and the heat acquired by the cold body are equal.
Heat gain = Heat lost
i.e., the rule of conservation of thermal energy governs the principle of calorimetry.
Using the following formula, the heat transfer in a system is determined.
q = mc∆t
where;
q = measure of heat transfer
m = mass of the body
c = specific heat of the body
Δt = change in the temperature
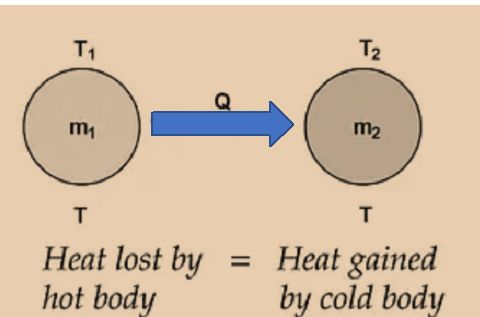
If two substances having masses m1 and m2, specific heats c1 and c2 at temperatures T1 and T2 (T1 >T2) are mixed.
Hence, temperature of mixture at equilibrium is Tmix.
Then,
m1c1 (T1 – Tmix) = m2c2 (Tmix – T2)
The exchange of heat occurs when two bodies with different surface temperatures come into thermal contact. At higher temperatures, the body emits heat, and at lower temperatures, the body receives heat. This back-and-forth will continue until they reach thermal equilibrium. Heat is released by the body at higher temperatures, whereas heat is absorbed by the body at lower temperatures. The concept of heat energy as a measurement of change in body temperature evolved much later, after a series of studies were carried out using calorimeters.
Assumptions in Calorimetry
- There is no heat transfer between the calorimeter and the surrounding environment.
- No heat is absorbed or released by the calorimeter materials.
- Pure water has the same density and specific heat capacity as a dilute aqueous solution.
Types of Calorimetry
Direct calorimetry: It detects the heat change of a chemical reaction by directly measuring the temperature change it creates.
Indirect Calorimetry: It is a way of evaluating an organism’s heat change by monitoring either its intake of oxygen or its production of carbon dioxide or nitrogen.
Differential Scanning Calorimetry: Differential scanning calorimetry is a technique for determining the amount of energy required to elevate the temperatures of a sample and a reference substance by the same amount. It is frequently used to determine the specific heat capacity of different proteins and other biological components, as well as to explore their reaction to heating.
These techniques are most applicable in biology for the study of heat transfer in organisms.
Applications of Calorimetry
Calorimetry, as a thermal analysis technique, has a broad range of applications that include not only studying the thermal characterisation (e.g. melting temperature, denaturation temperature, and enthalpy change) of small and large drug molecules, but also characterisation of fuel, metals, and oils.
- It is used to calculate the enthalpy changes that occur during a reaction.
- It is frequently used to investigate pharmacological and biological molecule thermal properties, such as denaturation temperature.
- This is also used to analyze polymers, allowing us to determine parameters such as crystallisation temperature.
- Generally used in food laboratories to determine the calorie content of foods.
- It is also used to determine the thermal characteristics of medicines, proteins, and other biological substances.
Calculating Enthalpy change using Calorimetry
By using a method calorimetry, we are able to determine the enthalpy change of various reactions.
A polystyrene cup can serve as a calorimeter.
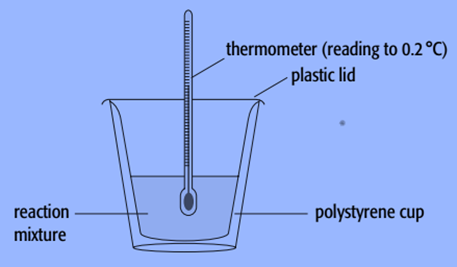
We employ known amounts of reactants and known volumes of liquids while doing experiments with calorimeters. As the reaction takes place, we also track the liquid’s temperature change in the calorimeter.
The energy is transferred as heat (the enthalpy change) is given by the relationship:
ΔH = –mcΔT
where:
ΔH is the enthalpy change, in J
m is the mass of water, in g
c is the specific heat capacity, in J g–1 °C–1
ΔT is the temperature change, in °C
Steps to determine enthalpy of the reaction
- A polystyrene cup can be used as a calorimeter to determine the enthalpy change of a sodium hydroxide solution. To ensure that all of the solute dissolves, we employ known amounts of solvent and solute with an excess of the solvent.
- Weigh an empty polystyrene cup first.
- Fill the cup with 100cm3 of water, then weigh the contents together.
- Use a thermometer to measure the water’s constant temperature, preferably to within 0.2 °C.
- Add a few sodium hydroxide pellets that have been dried-stored.
- Continue to stir the mixture while using a thermometer, and record the temperature at regular intervals, such as every 20 seconds.
- After the maximum temperature has been attained, continue to record the temperature for a further five minutes.
- Weigh the cup and everything inside it to determine how much sodium hydroxide dissolved.
Enthalpy change (J) of Sodium Hydroxide;
= – mass of water (g) × specific heat capacity (Jg–1 °C–1)× temperature change (°C)
Calculating the Enthalpy Change of Combustion
By burning a known mass of material and utilizing the heat generated to raise the temperature of a known mass of water, we may determine the enthalpy change of combustion. A metal calorimeter and a spirit burner are the tools used for this.
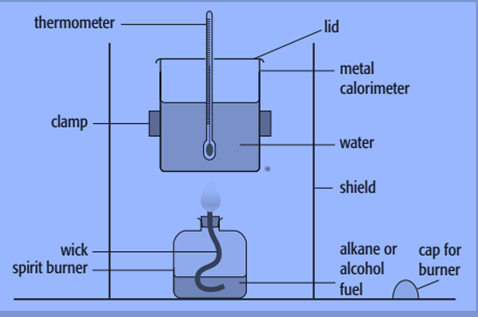
Steps to determine enthalpy of the combustion of propan-1-ol
- Weigh the propan-1-ol containing spirit burner. When the burner is not lit, the cap must be maintained to prevent fuel evaporation.
- Fill up the calorimeter with 100 cm3 (100g) of water. This needs to be balanced out for improved accuracy.
- Stir the water and use a to take a temperature reading and the reading must be accurate to at least 0.1 °C.
- Position the spirit burner underneath the thermometer and light the wick after removing the cap. The size of the wick should have been modified in the past so that the wick’s substance doesn’t burn, and the flame barely reaches the calorimeter’s base.
- Continue agitating the water while using a thermometer until there is a 10 °C spike in temperature. Record this temperature.
- Remove the spirit burner, place the cap on it and reweigh it.
Using the relationship ΔH = –mcΔT (mass of water × specific heat capacity of water × temperature change)
Calculate the energy released by burning propan-1-ol.
Limitation
Calorimetry can be quite difficult. This is because there are numerous variables at work during calorimetry, and it is hard to correctly monitor all of them.
- Energy may be transferred to or away from the environment, typically in the form of heat loss.
- We frequently presume that the used solution has the density and specific heat capacity of pure water, although this may not always be the case.
- The reaction might not be fully complete.
- The apparatus could be heated instead of the solution by some of the heat energy generated.
- Possible evaporation of some of the fuel
References
- Atkins, Peter and de Paula, Julio; Physical Chemistry for the Life Sciences, United States, 2006.Katherine Hurley
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/Calorimetry
- https://www.studysmarter.us/explanations/chemistry/physical-chemistry/calorimetry/
- https://byjus.com/physics/principle-of-calorimetry/
- Bryan, G.H. (1907). Thermodynamics. An Introductory Treatise dealing mainly with First Principles and their Direct Applications, B.G. Tuebner, Leipzig.
- Crawford, F.H. (1963). Heat, Thermodynamics, and Statistical Physics, Rupert Hart-Davis, London, Harcourt, Brace, & World.
