Calcium is a chemical element that belongs to Group 2 (IIa) of the periodic table and is represented as (Ca) in the periodic table. It is the fifth most plentiful element in the earth’s crust and the most abundant metallic element in the human body. It is the 20th element in the periodic table.
The metal is trimorphic, which means it is tougher than sodium but softer than aluminum. It has the same properties as beryllium and aluminum, and unlike alkaline metals, it does not cause skin burns. It has a lower chemical reactivity than alkaline metals and alkaline-earth metals. Calcium does not exist in its pure form, however calcium compounds are extensively dispersed.
History of Calcium
- Calcium compounds have been known for millennia, but their chemical composition was not discovered until the 17th century.
- Lime was utilized as a construction material and as plaster for statues as early as 7000 BC.
- The first dated lime kiln dates back to 2500 BC and was found in Khafajah, Mesopotamia.
- Dehydrated gypsum (CaSO4.2H2O) was utilized on the Great Pyramid of Giza at about the same period.
- The ancient Romans instead used lime mortars made by heating limestone (CaCO3). The name “calcium” itself derives from the Latin word calx “lime”.
- Vitruvius, a Roman architect and engineer who lived in the first century BC, observed that the resulting lime was lighter than the initial limestone, which he attributed to the boiling of the water.
- In 1755, Joseph Black proved that this was due to the loss of carbon dioxide, which as a gas had not been recognized by the ancient Romans.
Calcium, along with its congeners magnesium, strontium, and barium, was first isolated by Humphry Davy in 1808.
Occurrence of Calcium
Despite being the fifth most prevalent element in the earth’s crust, calcium is never found free in nature because it rapidly creates compounds when it reacts with oxygen and water.
- Calcium makes up 3.64 percent of the Earth’s crust and 8% of the Moon’s crust, and its cosmic abundance is calculated to be 4.9 ×104 atoms.
- On Earth, it is found in limestone, chalk, marble, dolomite, eggshells, pearls, coral, stalactites, stalagmites, and the shells of many marine animals.
- It is the main inorganic ingredient of teeth and bones as calcium hydroxyl phosphate and occurs as the mineral apatite.
The major producers of calcium are China (about 10000 to 12000 tonnes per year), Russia (about 6000 to 8000 tonnes per year), and the United States (about 2000 to 4000 tonnes per year). Canada and France are also among the minor producers.
Some of the important calcium sources in nature:
- apatites
- alabaster
- plaster
- calcite
- fluorite
- dolomite
Isotopes of Calcium
Calcium has 19 Isotopes whose half-lives are known, with mass numbers 35 to 53. Naturally occurring calcium is a mixture of six isotopes and they are found in the percentages shown: 40Ca (97%), 42Ca (0.6%), 43Ca (0.1%), 44Ca (2%), 46Ca (0.004%), and 48Ca (0.2%).
Naturally occurring isotopes and their natural abundance
| Isotope | Natural abundance (atom %) |
|---|---|
| 40Ca | 96.941 (156) |
| 42Ca | 0.647 (23) |
| 43Ca | 0.135 (10) |
| 44Ca | 2.086 (110) |
| 46Ca | 0.004 (3) |
| 48Ca | 0.187 (21) |
Calcium isotopes (primarily Ca-42, Ca-44, Ca-46, and Ca-48) are widely utilized in clinical research, particularly in nutritional investigations. They are mostly used to assess calcium absorption in women and children. Calcium deficiency is significantly linked to the progression of osteoporosis in adults. Calcium insufficiency is mostly linked to the development of rickets in children.
Ca-48 has been used to bombard Pb and Bi targets in order to produce super-heavy elements.
Elemental Properties of Calcium
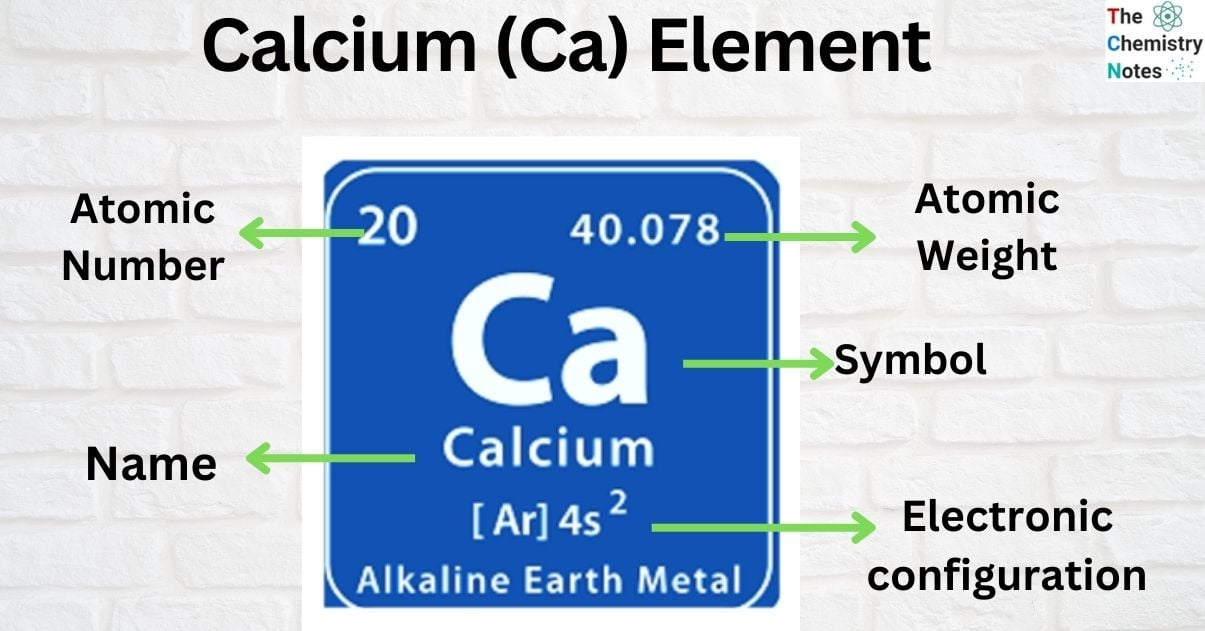
| Electronic Configuration | [ Ar ] 4s2 |
| Atomic Number | 20 |
| Atomic Weight | 40.08 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 2, 4, s-block |
| Density | 1.55 g.cm -3 at 20 °C |
| Ionic radius | 0.099 nm |
| Van der Waals radius | 0.197 nm |
| Electron shells | 2,8,8,2 |
| Electrons | 20 |
| Protons | 20 |
| Neutrons in most abundant isotope | 20 |
Physical Properties of Calcium
- Calcium is a fairly soft metal with a shiny silver surface when first cut.
- It has a higher melting temperature than alkali metals and is produced as a face-centered cubic crystal lattice.
- Compared to the preceding noble gas argon, it has a 4s2 valence shell electronic arrangement. Inclusion into Group-2 or IIA of the periodic table results from the s2 configuration.
- It is used to alloy aluminum, lead, copper, and other base metals.
- Calcium has a density of 1.55 grams per cubic centimeter. It has a melting point of 842°C and a boiling point of 1484°C.
- It is a soft metal.
- Calcium is a good conductor.
- It is malleable and ductile in nature.
- The surface quickly becomes dull as calcium reacts with oxygen to form a coating of white or gray calcium oxide.
| Color/physical appearance | Silvery-white metallic |
| Melting point/freezing point | 842°C, 1548°F, 1115 K |
| Boiling point | 1484°C, 2703°F, 1757 K |
| Density | 1.54 (g cm−3) |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1 (Pauling Scale) 1.034 (Allen Scale) |
Chemical Properties of Calcium
- Calcium is a moderately active element. It reacts readily with oxygen to form calcium oxide (CaO).
- Calcium ignites when heated in air or oxygen.
- Unlike other alkaline metals, calcium is less chemically reactive and doesn’t burn skin.
- Calcium combines with other elements to form certain compounds that are abundant in the earth’s crust. Some of the natural forms of calcium are: limestone (CaCo3), fluorite (CaF2) and gypsum (CaSO4·2H2O).
- When calcium comes in contact with air, it forms a coating of nitride and oxide to minimize further corrosion. When subjected to very high temperature in the air, calcium can burn to produce nitride.
Chemical Reaction of Calcium
Reaction of Calcium with air
Calcium is a white metal that has a silvery luster. The surface of calcium metal is coated with a thin layer of oxide, which protects it from air assault to a smaller extent than the similar layer in magnesium. When calcium metal is lit, it burns in the air, producing a combination of white calcium oxide, CaO, and calcium nitride, Ca3N2. Calcium oxide is typically produced by heating calcium carbonate. Calcium is more reactive with air than magnesium, which is located just below it on the periodic table.
2Ca(s) + O2(g) → 2CaO(s)
3Ca(s) + N2(g) → Ca3N2(s)
Reaction of Calcium with water
Calcium has a delayed reaction with water. In contrast, magnesium, which comes directly above calcium in the periodic table, is essentially unreactive with cold water. Calcium hydroxide, Ca(OH)2, and hydrogen gas (H2) are formed as a result of the process. The calcium metal sinks in water, and after an hour or two, bubbles of hydrogen are visible on the metal’s surface.
Ca(s) + 2H2O(g) → Ca(OH)2(aq) + H2(g)
Reaction of Calcium with halogens
Calcium reacts strongly with the halogens fluorine, F2, chlorine, Cl2, bromine, Br2, or iodine, I2 to create the dihalides calcium(II) fluoride, CaF2, calcium(II) chloride, CaCl2, calcium(II) bromide, CaBr2, and calcium(II) iodide, CaI2.
Heat is required for the creation of the products in bromine and iodine reactions.
Ca(s) + F2(g) → CaF2(s)
Ca(s) + Cl2(g) → CaCl2(s)
Ca(s) + Br2(g) → CaBr2(s)
Ca(s) + I2(g) → CaI2(s)
Reaction of calcium with acids
Calcium metal dissolves readily in dilute or concentrated hydrochloric acid to form solutions containing the aquated Ca(II) ion together with hydrogen gas, H2.
Ca(s) + 2HCl(aq) → Ca2+(aq) + 2Cl–(aq) + H2(g)
Uses of Calcium
- Calcium forms alloys with aluminum, beryllium, copper, lead, and magnesium.
- Calcium is also used to make alloys. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. An alloy of calcium and cerium is used in flints found in lighters (the elements that create sparks).
- In the steel industry, it is used as a scavenger to remove oxygen, sulfur, and phosphorus.
- The biological use of calcium is to provide strength and structure to the skeleton. It is vital for the maintenance of bones and teeth.
- It is used in the manufacture of other metals such as uranium and thorium.
- Calcium from limestone is a vital component of Portland cement.
- Gypsum (calcium sulfate) is used by builders as a plaster and by nurses for setting bones, as ‘plaster of Paris’.
- The element assists in the movement of muscles by helping to carry messages from the brain to all parts of the body.
- The largest use of metallic calcium is in steelmaking, due to its strong chemical affinity for oxygen and sulfur. Its oxides and sulfides, once formed, give liquid lime aluminate and sulfide inclusions in steel which float out; on treatment, these inclusions disperse throughout the steel and become small and spherical, improving castability, cleanliness and general mechanical properties.
- As a food additive, calcium concatenate is utilized.
- Calcium carbide is used to make polymers and acetylene gas.
- Many calcium compounds are used in food, as pharmaceuticals, and in medicine, among others. For example, calcium and phosphorus are supplemented in foods through the addition of calcium lactate, calcium diphosphate, and tricalcium phosphate.
- In baking, calcium phosphate is used as a leavening agent.
- Giving calcium gluconate intravenously (IV) can correct hyperkalemia, a condition in which the blood contains too much potassium.
- Quicklime (CaO) is used in many applications in the chemical industry, such as treatment of drinking water – especially for water softening and arsenic removal, animal waste and wastewater.
Health Effects of Calcium
- Calcium is the most abundant metal in the human body: is the main constituent of bones and teeth and it has keys metabolic functions.
- It is frequently found in milk and milk products, but it may also be found in vegetables, nuts, and beans. It is required for the preservation of the human bones and teeth. It also helps neurons and muscles work.
- More than 2,5 grams of calcium per day without a medical requirement might cause kidney stones and sclerosis of the kidneys and blood vessels.
- One of the main causes of osteoporosis is a lack of calcium. Osteoporosis is a condition in which the bones become extremely porous, prone to fracture, and mend slowly, affecting mostly women after menopause and frequently resulting in spinal curvature due to vertebral collapse.
- Ca2+ may boost high-density lipoprotein, prevent colon polyps, lower blood pressure, and aid in weight reduction.
- Dairy products are the most common calcium sources, although nuts, certain green vegetables including spinach and cauliflower, beans, and lentils are also good sources.
- Endothelial cells respond to stimulus with precisely calibrated signaling responses during angiogenesis, and Ca2+ plays a role in angiogenesis control.
- Mutations or functional anomalies in the different Ca2+ transporters can result in a variety of illnesses.
- Mutations in ryanodine receptors (malignant hyperthermia, porcine stress syndrome, and central core disease), dihydropyridine receptors (familial hypokalemic periodic paralysis, malignant hyperthermia, muscular dysgenesis), or Ca2+ pumps (Brody disease) can induce skeletal-muscle pathology.
Environmental Effects of Calcium
Calcium itself is not harmful to humans or the environment but Calcium phosphide is very toxic to aquatic organisms. Soil with an excess of calcium can cause the pH to be excessively high (above 7), or alkaline, which sometimes reduces the solubility of nutrients such as phosphate and many micronutrients, limiting plant growth.
Biological Importance of Calcium
Calcium is one of the most essential and abundant elements in the human body. It is connected with healthy bones and teeth, helps muscles contract, and plays a key part in blood clotting.
- Calcium is mostly present in the bones and teeth of living creatures.
- This mineral is found in huge quantities in blood. It aids in blood coagulation. Calcium deficiency prolongs the clotting time of the blood.
- Calcium promotes muscular contraction.
- Ca2+ signaling is vital in exocrine and hormone secretion, muscular and non-muscular movement, and the activation and control of various metabolic processes. Calcium transporting systems in the plasma membrane and organelles regulate the ionic concentration of calcium in various compartments according to the different demands of the physiological cycle, and these systems upregulate calcium entry through the action of several hormones and calcium binding proteins.
- It is important in the nitrogen metabolism of plants. The absence of this mineral in plants impacts the size and quantity of chloroplasts.
- The concentration of cytoplasmic calcium (Ca2+) influences several cellular activities, including gene expression, metabolism, proliferation, secretion, neuronal stimulation, and fertilization.
- The disruption of mitochondrial Ca2+ homeostasis is now recognized as a crucial factor in various illnesses that increase reactive oxygen species formation, pore triggering, and cytochrome c release, ultimately leading to death.
Fun Facts About Calcium
- Lime is calcium oxide, which produces a brilliant, intense light when burnt in an oxyhydrogen flame. It was used to light the stage in theaters during the 1800s until electricity took over.
- Cells in animals and plants must communicate with other cells. This is called signaling. Calcium ions are the most important messengers between cells in living things and are absolutely vital for the existence of multicellular life forms.
- Vitamin D is essential for calcium absorption by the human body. Vitamin D is converted to a hormone that causes intestinal proteins responsible for calcium absorption to be produced.
- In comparison to omnivores and vegetarians, vegans with calcium intakes more than 525 mg/day had no change in fracture risk.
- The Egyptians used limestone stones to construct their pyramids. Limestone is nothing more than calcium carbonate crystallized.
Watch out the video to learn more about calcium.
References
- Lavoisier, Antoine; Kerr, Robert (translator) (1799) Elements of Chemistry, 4th ed. Edinburgh, Scotland: William Creech
- John Davy, Memoirs of The Life of Sir Humphry Davy., Vol 1, 1836, p395, Longman.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- https://www.geeksforgeeks.org/potassium-and-calcium-atomic-structure-chemical-properties-uses/
- https://www.lenntech.com/periodic/elements/ca.htm
- https://www.britannica.com/science/calcium#ref1017
- C. R. Hammond The elements (pp. 4–35) in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics
- https://byjus.com/chemistry/calcium/
- https://www.rsc.org/periodic-table/element/20/calcium
- https://pubchem.ncbi.nlm.nih.gov/element/Calcium#section=Ionization-Energy
- https://medlineplus.gov/ency/article/002412.htm#:~:text=Calcium%20is%20one%20of%20the,lifetime%20can%20help%20prevent%20osteoporosis.

