Buffer solutions are also useful in chemical and biochemical processes where pH control is critical. A constant pH is required for many biological and chemical reactions to proceed. In these systems, buffers are extremely useful to maintain pH. Buffers are extremely useful in these systems for keeping the pH constant. This does not imply that the pH of buffers remains constant. Simply put, it indicates that the pH change is not as significant as it would be in the absence of a buffer-containing solution.

What is Buffer Solution?
Buffer Solution is a water-based solvent-based solution composed of a weak acid and its conjugate base, or a weak base and its conjugate acid. They are resistant to pH changes caused by dilution or the addition of small amounts of acid/alkali to them.
The pH of Buffer Solutions changes very little when a very small amount of strong acid or strong base is added. As a result, they are used to keep the pH constant.
In other words, a buffer solution (also known as a pH buffer or hydrogen ion buffer) is an aqueous solution composed of a weak acid and its conjugate base, or vice versa. Buffer solutions are used in a wide range of chemical applications to maintain a nearly constant pH. Many living systems in nature use buffering to regulate their pH.
For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean.
Types of Buffer Solutions
Buffer solutions are broadly classified into two types: acidic buffers and alkaline buffers.
Acidic Buffer
Acid buffer has a pH of acid and is made by combining a weak acid and its salt with a strong base.
An acidic buffer has a pH of less than 7 and is composed of a weak acid and its conjugate base as a salt. If you want to change the pH of the solution, you can adjust the acid-salt ratio. Furthermore, different acids (along with their conjugate salts) can have varying effects on pH.
A mixture of sodium acetate and acetic acid (pH = 4.75) is an example of an acidic buffer solution.
CH3COOH (aq) ⇄ CH3COO–(aq) + H+(aq)
Acetic acid and sodium acetate is an example of a weak acid and its conjugate salt. The acid equilibrium is shifting to the left; however, if sodium acetate is added, acetate ions are introduced into the solution. The equilibrium will then shift to the left due to Le Chatelier’s Principle.
Alkaline Buffer
A basic buffer is a solution with a pH greater than 7 that is made from a weak base and its conjugate acid as a salt.
To begin, the concentrations of both components should be equal; however, as with acidic buffers, you can change the pH of the solution by varying the ratio of base to acidic salt. These buffer solutions are used to keep basic conditions stable. The pH of the basic buffer is basic, and it is made by combining a weak base and its salt with a strong acid. The pH of an aqueous solution containing equal amounts of ammonium hydroxide and ammonium chloride is 9.25.
Examples of a weak base and its conjugate acid include ammonia and ammonium chloride.
NH3(aq) + H2O(l) ⇄ NH4+(aq) + OH–(aq)
The equilibrium is shifting to the left; however, if ammonium chloride is added, ammonium ions are introduced into the solution. This time, Le Chatelier’s Principles will cause the equilibrium to shift to the left even further.
Principles of buffering
An equilibrium between the weak acid HA and its conjugate base A causes buffer solutions to resist pH change:
HA ⇌ H+ + A−
In accordance with Le Chatelier’s principle, when some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added and the equilibrium is shifted to the left. As a result, the hydrogen ion concentration rises less than expected for the amount of strong acid added. Similarly, adding strong alkali to the mixture reduces the hydrogen ion concentration by less than the amount expected for the amount of alkali added.
The reaction consumes the majority of the added hydroxide ion, causing the hydrogen ion concentration to drop less than anticipated.
OH− + HA → H2O + A−
with only a small amount consumed in the neutralization reaction (which is the reaction that results in an increase in pH)
OH− + H+ → H2O
When the acid has been deprotonated to more than 95%, the pH rises rapidly because the majority of the added alkali is consumed in the neutralization reaction.
Buffer Solution Examples
- Acetic acid & conjugate base: CH3COOH & CH3COO–
- Formic acid & conjugate base: HCHO2 & CHO2–
- Pyridine & conjugate acid: C5H5N & C5H5H+
- Ammonia & conjugate acid: NH3 & NH4+
- Methylamine & conjugate acid: CH3NH2 & CH3NH3+
Properties of Buffer
- Buffer solutions are known to be resistant to pH changes. A buffer solution’s pH, on the other hand, can vary depending on how much strong acid or strong base is added.
- The amount of strong acid or base in the buffer solution, as well as the buffer solution’s core components, all have an effect on buffer capacity.
- The capacity of buffer solutions is equal to the amount of base when a strong acid is added. When a strong base is added, the capacity is equal to the amount of acid.
- A buffer is a water-based solution containing an acid and either its conjugate base or its conjugate acid. Due to the weak nature of the acids and bases in a buffer, adding a small amount of a strong acid or base has little effect on the pH
How buffer solutions work?
The equilibria help us understand how a buffer solution works involved.
Ethanoic acid is an extremely weak acid. As a result, it remains mostly unionized (CH3COOH) and only produces a low concentration of ethanoate ions in solution:
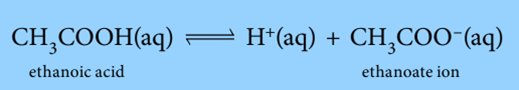
Sodium ethanoate is fully ionized in an aqueous solution:
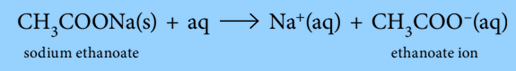
Both CH3COOH and CH3COO– are present in relatively high concentrations in the buffer solution. The acid (CH3COOH) and its conjugate base are said to have reserve supplies (CH3COO–). The pH of a buffer solution is determined by the concentration ratio of the acid and its conjugate base. If this does not change significantly, the pH will change very little.
Example
Ethanoic acid molecules in the buffer solution are in equilibrium with hydrogen ions and ethanoate ions:
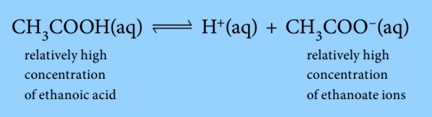
This equation can be used to explain how buffer solutions work.
A rise in hydrogen ion concentration would significantly lower water’s pH, but when H+ ions are added to the buffer solution:
- Because H+ ions combine with CH3COO– ions to form more CH3COOH until equilibrium is re-established, the addition of H+ ions shifts the position of equilibrium to the left. The large reserve supply of CH3COO– ensures that the concentration of CH3COO– ions in the solution does not change significantly.
- Because there is a large reserve supply of CH3COOH, the concentration of CH3COOH molecules in the solution does not change significantly, and thus the pH does not change significantly.
An increase in hydroxide ion concentration would greatly increase the pH of water, but when OH– ions are added to the buffer solution:
- the added OH– ions combine with H+ ions to form water
- this reduces the H+ ion concentration
- the position of equilibrium shifts to the right
- so CH3COOH molecules ionise to form more H+ and CH3COO– ions until equilibrium is re-established
- the large reserve supply of CH3COOH ensures that the concentration of CH3COOH molecules in the solution does not change significantly
- the large reserve supply of CH3COO– ensures that the concentration of CH3COO– ions in solution does not change significantly
- so the pH does not change significantly.
Preparation of Base Buffer Solution
Consider a basic buffer solution with strong acid, salt (BA), and a weak base (B).
As a result, the basic buffer solution will be,
pOH = pKb + log ([salt]/[base])
pOH of a basic buffer solution = pKb + log ([salt]/[acid])
pH of a basic buffer solution = pKa – log ([salt]/[acid])
If the acid’s (pKa) and base’s (pKb) dissociation constants are known, a buffer solution can be prepared by controlling the salt-acid or salt-base ratio.
As previously stated, these solutions are made by combining weak bases with their corresponding conjugate acids, or weak acids with their corresponding weak bases.
The preparation of a phosphate buffer by mixing hydrogen phosphate HPO42- and dihydrogen phosphate H2PO4– is an example of this method of preparing buffer solutions. This solution maintains a pH of 7.4.
Buffer Capacity
The ability of a buffer to resist a change in pH caused by the addition of a small amount of strong acid or base is quantified as buffer capacity (β).
It is defined as,
β = db/d(pH)
or, β = − da/d(pH)
Where db and da are the moles of base or acid added to one liter of solution to change the buffer’s pH by a factor of one. As a result, buffer capacity is defined as the number of moles of acid or base required to change the pH of the buffer solution by one unit.
Uses of Buffer Solution
- Buffer solution is widely used in analytical chemistry, biological laboratories, and various industrial operations to maintain the desired pH range.
- Many laboratory reactions in analytical chemistry take place within a narrow pH range. Buffer solutions are frequently used in these situations to maintain the desired pH range. As a result, we used buffers in analytical chemistry for selective precipitation, solvent extraction, and compound titration.
- Buffers are used in chemistry to aid in the study of reaction rates at constant pH. The oxidation of iodide ions by hydrogen peroxide, for example, is a pH-dependent reaction. As a result, if the pH is kept constant, it is possible to investigate the effect of I– concentration.
- Buffing is required for colorimetric pH determinations, glass electrode standardization, and other emf measurements.
- Several operations in the industrial chemical process are pH controlled. As a result, we buffered such chemical processes in order to achieve the best results. Metal electrodeposition, leather tanning, alcohol brewing, paper manufacturing, and other such processes are examples.
- Buffering actions are extremely beneficial in biological systems. pH is highly selective in enzyme catalyzed reactions. As a result, the buffers maintain the effective pH range of the enzyme-catalyzed reaction.
- A buffer solution of carbonic acid and sodium carbonate maintains the pH of human blood between 7.3 and 7.5.
References
- Skoog, Douglas A.; West, Donald M.; Holler, F. James; Crouch, Stanley R. (2014). Fundamentals of Analytical Chemistry (9th ed.)
- Butler, J. N. (1998). Ionic Equilibrium: Solubility and pH calculations.
- https://byjus.com/jee/buffer-solutions/#Uses-of-Buffer-Solutions
- https://www.geeksforgeeks.org/buffer-solutions-definition-types-preparation-uses/
- https://www.priyamstudycentre.com/2022/01/buffer-solution.html
- https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/buffer-solution/9059/
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/buffer-solutions/
- https://courses.lumenlearning.com/chemistryformajors/chapter/buffers-2/
- https://www.chemicals.co.uk/blog/uses-buffer-solutions#:~:text=Buffer%20solutions%20are%20used%20in%20the%20manufacture%20of%20many%20cosmetic,this%20could%20cause%20skin%20irritations.
