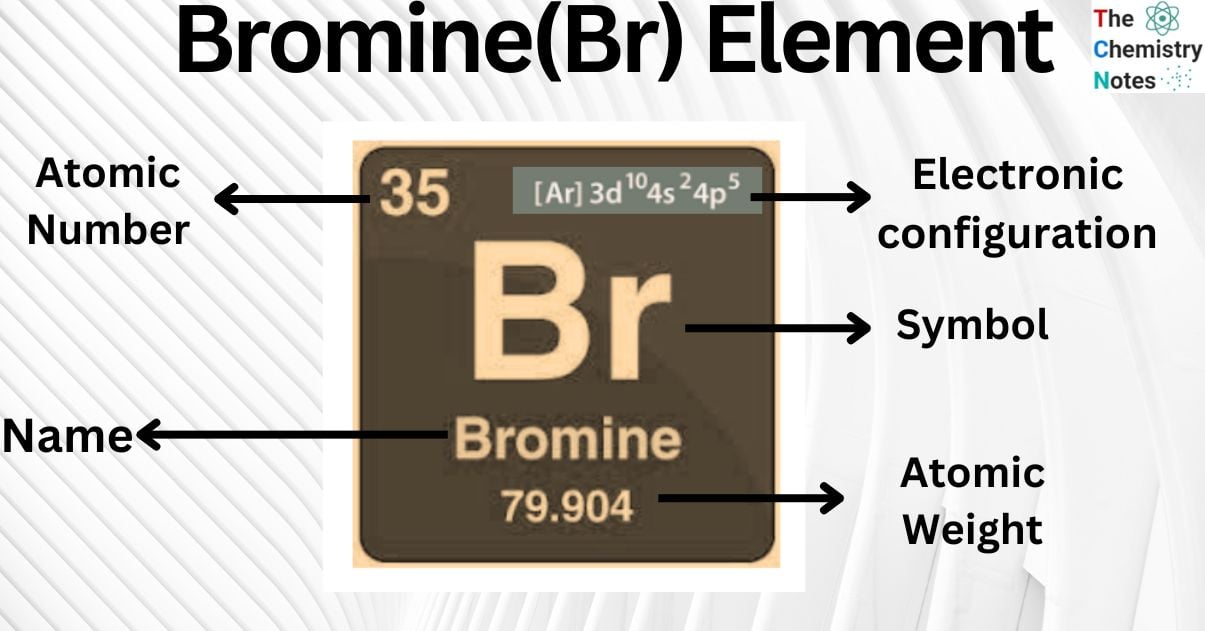
Bromine is a metallic element with the atomic number 35 and is represented by the symbol ‘Br’ in the periodic table. It is classified as a halogen and belongs to the p-block of group 17 of the periodic table.
Bromine is in a liquid state at room temperature. It is a brownish-red liquid. It has properties similar to those of fluorine, chlorine, and iodine.
History of Bromine
- In 1825, a salt producer requested that Justus von Liebig, a German chemist, analyze a sample of salt spring water from the German town of Bad Kreuznach. He discovered bromine during his experiment with the sample but mistook it for iodine.
- Carl Jacob Lowig, a German chemist, produced bromine for the very first time in 1825 as a young chemistry student before his freshman year at Heidelberg.
- Unfortunately, because Lowig’s study was delayed, another scientist was able to publish a report on bromine in 1826, making him the discoverer of this new element.
- Antoine-Jerome Balard (French chemist) discovered Bromine in 1826.
- Balard identified bromine compounds in the ash of seaweed from the salt marshes of Montpellier. Although the seaweed was utilized in the production of iodine, it also contained bromine. Balard recovered bromine from a chlorine-saturated seaweed ash solution.
- Because of the element’s foul odor, the French Academy of Sciences proposed the name bromine, derived from the Greek word bromos, which means “bad smell” or “stench.”
Occurrence of Bromine
- Bromine is the 46th most prevalent element in the Earth’s crust and is far less common in the crust than fluorine or chlorine. It is a rare element, is found in nature and spread throughout the Earth’s crust only in compounds known as soluble and insoluble bromides.
- Bromine can primarily be recovered from soluble salts found in seawater, salt lakes, inland seas, and brine wells.
- Israel, Ukraine, and the United Kingdom are among the biggest producers of bromine, with a combined production of more than 300 thousand metric tons per year.
Isotopes of Bromine
Bromine has two naturally occurring stable isotopes: 79Br and 81Br,
Naturally occurring isotopes
| Isotopes | Natural abundance (atom %) |
|---|---|
| 79Br | 50.69 (7) |
| 81Br | 49.31 (7) |
Elemental Properties of Bromine
| Electronic Configuration | [Ar] 3d104s24p5 |
| Atomic Number | 35 |
| Atomic Weight | 79.904 g.mol -1 |
| State at 20°C | Liquid |
| Group, Period, and Block | 17, 4, p-block |
| Density | 3.1028 g.cm -3 at 20 °C |
| Ionic radius | 0.195 nm (-1) |
| Van der Waals radius | 0.165 nm |
| Electron shells | 2, 8, 18, 7 |
| Electrons | 35 |
| Protons | 35 |
| Neutrons in most abundant isotope | 44 |
Physical Properties of Bromine
- Bromine has an atomic number of 35 and is a non-metal that is brownish-red in color and liquid at room temperature.
- The density of Bromine is 3.10 grams per cubic centimeter.
- It has a melting point of −7.2°C (19°F) and a boiling point of 58.8°C (137.8°F).
- Bromine has a pungent odor.
| Color/physical appearance | Red-brown/ Liquid |
| Melting point/freezing point | −7.2°C, 19°F, 266 K |
| Boiling point | 58.8°C, 137.8°F, 332 K |
| Density | 3.1028 g cm-3 at 20°C |
| Electronegativity | 2.8 [Pauling Scale] |
Chemical Properties of Bromine
Bromine has a considerably higher electron affinity than chlorine. However, because the bromide ion has lesser solubility than the chloride ion, its oxidizing ability is comparatively low.
Chemical Reaction Of Bromine
- Reaction of Bromine with Water
When bromine, Br2, interacts with water, hypobromite, OBr–, is formed. The position of the equilibrium is highly dependent on the pH of the solution.
Br2 (l) + H2O (l) ⇌ OBr– (aq) + 2H+ (aq) + Br– (aq)
- Reaction of Bromine with Air
Bromine, Br2, does not react with oxygen, O2, or nitrogen, N2. However, around -78°C, bromine reacts with ozone, O3, which is the second allotrope of oxygen, to generate the unstable dioxide bromine(IV) oxide, BrO2.
Br2 (l) + 2O3 (g) → O2 (g) + 2BrO2 (s) [brown]
- Reaction of Bromine with the Halogens
Bromine, Br2, interacts with fluorine, F2, in the gas phase to generate the interhalogen species BrF. Because BrF disproportionately reacts at room temperature to create bromine, Br2, Br3, and BrF5, it is hard to acquire in its pure form.
Br2 (g) + F2 (g) → 2BrF (g)
3BrF (g) → Br2 (l) + BrF3 (l)
5BrF (g) → 2Br2 (l) + BrF5 (l)
Excess fluorine, F2, combines with bromine, Br2, to generate the interhalogen molecule BrF5 at approximately 150 °C.
Br2 (l) + 5F2 (g) → 2BrF5 (l)
In the gas phase, chlorine, Cl2, combines with bromine, Br2, to generate the unstable interhalogen species bromine (I) chloride, ClBr.
Cl2 (g) + Br2 (g) → 2ClBr (g)
At normal temperature, bromine, Br2, combines with iodine, I2, to generate the interhalogen species bromine(I) iodide, BrI.
Br2 (l) + I2 (s) → 2IBr (s)
- Reaction of Bromine with Bases
Bromate, BrO3–, is formed when bromine, Br2, interacts with hot aqueous alkali. In this process, just one-sixth of the total bromine is transformed.
3Br2 (g) + 6OH– (aq) → BrO3– (aq) + 5Br– (aq) + 3H2O
Uses Of Bromine
Since the discovery of bromine, its compounds have found widespread use in a variety of industries. some of which are discussed below:
Used For Water Purification: Bromine is a powerful disinfectant. In water, bromine produces hypobromous acid (HOBr), a disinfectant that destroys waterborne bacteria. As a result, it is used as a substitute for chlorine in swimming pools and spas to disinfect water and remove bacteria, viruses, fungi, and other pathogens. It also aids in the prevention of algae and bacterial growth in industrial processes. It also imparts a medicinal taste to water. As a result, bromine should only be used to treat
Used For Photography Film: Silver bromide (AgBr) is a water-insoluble salt with exceptional light sensitivity. Bromine compounds are utilized to create a light-sensitive component of a photographic emulsion. Because of their photosensitivity, bromine is employed in the film business. Without silver bromide, the image would be too blurry or distorted.
Used In Pesticides: Although bromine is harmful to the human body, it is also widely used in pesticides. It is effective at controlling pest infestations. Bromine chemicals have long been used to eliminate pests in both the earth as well as in grain.
Used In Batteries: Bromine is also used in a variety of energy applications. Bromine-based storage batteries are extremely cost-effective and efficient, offering a variety of alternatives for utilizing renewable energy sources and managing energy. Bromine batteries include zinc bromide (ZnBr2) and hydrogen bromide (HBr). Grids and backup storage are common applications.
Used In Rubber: For decades, bromine has been used to make rubber. By combining bromine with butyl rubber, bromobutyl rubber is created. It improves its capacity to attach to metals, maintain stability at high temperatures, and resist weather, shock, and aging. It is also used to make conveyor belts, seals, hoses, and tank linings.
Used In Medicine: Bromine has various pharmaceutical applications. Brominated chemicals are common ingredients in over-the-counter medications and other drugs such as tranquilizers, sedatives, and anti-epileptics. Some medications have been shown to be effective in treating pneumonia and cocaine addiction.
Used In Fire Resistor: One of the most common commercial applications of bromine is the production of bromine-based flame retardants (BFRs), which are chemical combinations added to various products to make them less combustible.BFRs are applied to textiles, plastics, and electrical equipment to make them flame retardants. They are typically included in these pieces during the production process.
Health Effects Of Bromine
- In liquid form, bromine is corrosive to human tissue, and its fumes irritate the eyes and throat. Bromine fumes are extremely poisonous when inhaled.
- The severity of bromine poisoning is determined by the amount, route, and length of time of exposure, as well as the person’s age and pre-existing medical condition.
- Swallowing bromine-containing compounds (a combination of bromide and other chemicals) might result in a variety of effects depending on the compound.
- Swallowing a considerable amount of bromine in a short period of time is likely to result in gastrointestinal symptoms such as nausea and vomiting.
- Organic bromines can also harm organs such as the liver, kidneys, lungs, and milt, as well as cause stomach and gastrointestinal problems. Some organic bromines, such as ethylene bromine, are carcinogenic.
Environmental Effects of Bromine
Bromine is toxic in nature and it has been known to have an impact on aquatic life.
- Acute animal tests in mice have produced LC50 values ranging from 240 ppm (1600 mg/m3; 2 hour exposure) to 750 ppm (5000 mg/m3; 7 minute exposure).
- After inhaling bromine vapours at 300 ppm (2000 mg/m3) for 3 hours, guinea pigs and rabbits developed pulmonary oedema, pseudomembranous deposits on the trachea and bronchi, and gastric mucosal bleeding. Animals who died several days after the exposure had bronchopneumonia and indications of functional abnormalities in the central nervous system.
- For four months, rats, mice, and rabbits inhaled 0.2 ppm (1.3 mg/m3) bromine and experienced respiratory, neurological, and endocrine system abnormalities. There were no harmful effects found at 0.02 ppm (0.13 mg/m3).
- Organic bromines are also harmful to mammals, particularly when they build up in the bodies of their prey. The most serious consequences on animals are nerve damage and DNA damage, which can increase the likelihood of cancer development.
Storage and Handling
- Keep the container well closed and in a cold, dry, well-ventilated place.
- To prevent leaking, open containers must be carefully resealed and kept upright. Polyethylene containers should not be used for storage. Use caution when handling and opening.
- Store away from direct sunlight.
- Avoid incompatible substances including reducing agents, alkali metals, powdered metals, iron, copper, stainless steel, aluminum, organic compounds, aldehydes, ketones, arsenic powder, amines, amides, phenols, alcohol, ammonia, and ozone. Bromine will degrade some polymers, rubbers, and coatings.
Interesting Facts about Bromine
- Bromine is utilized in water purification, pharmaceuticals, and sanitizers.
- Bromine is also used to lower mercury emissions from coal-fired power plants by up to 90%. The addition of bromine to the process oxidizes the mercury, making it easier to recover using emission control devices.
- The human body contains roughly 0.0004 percent bromine, however there is no recognized purpose for bromine in the human body.
- Bromide chemicals have historically been utilized as sedatives and anticonvulsants. In particular, sodium bromide and potassium bromide were employed in the nineteenth and twentieth centuries before being superseded by chloral hydrate, which was thereafter replaced by barbiturates and other medications.
Watch out the video about the unique liquid Bromine element.
References
- Mary Elvira Weeks, The discovery of the elements. XVII. The halogen family., J. Chem. Educ., 1932, 9 (11), p1915.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.121. ISBN 1-4398-5511-0.
- https://www.chemicool.com/elements/bromine.html
- https://byjus.com/chemistry/bromine/
- A.G. Ruaws, Pharmacokinetics of Bromide Ion — An Overview., Ed Chem. Toxic., 1983, vol. 21, 4 p379.
- J.W. Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry., 1922, vol 2, Longmans, Green and Co., p24.
- https://www.rsc.org/periodic-table/element/35/bromine
- https://www.lenntech.com/periodic/elements/br.htm
- https://pubchem.ncbi.nlm.nih.gov/compound/Bromine
- Ioffe, David and Kampf, Arieh (2002) “Bromine, Organic Compounds” in Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons. doi:10.1002/0471238961.0218151325150606.a01

