One of the most prevalent thermodynamic cycles found in gas turbine power plants or aircraft is the Brayton cycle. There are two broad categories of thermodynamic cycles: power cycles, which generate a net power output, and refrigeration and heat pump cycles, which require a net power input. Gas and vapor cycles are the two types of thermodynamic power cycles. The working fluid in gas cycles stays in the gas phase the entire time. In vapor cycles, the working fluid leaves as a liquid at one point in the cycle and as a vapor at another. Gas power cycle is used by gas turbines and internal combustion engines.
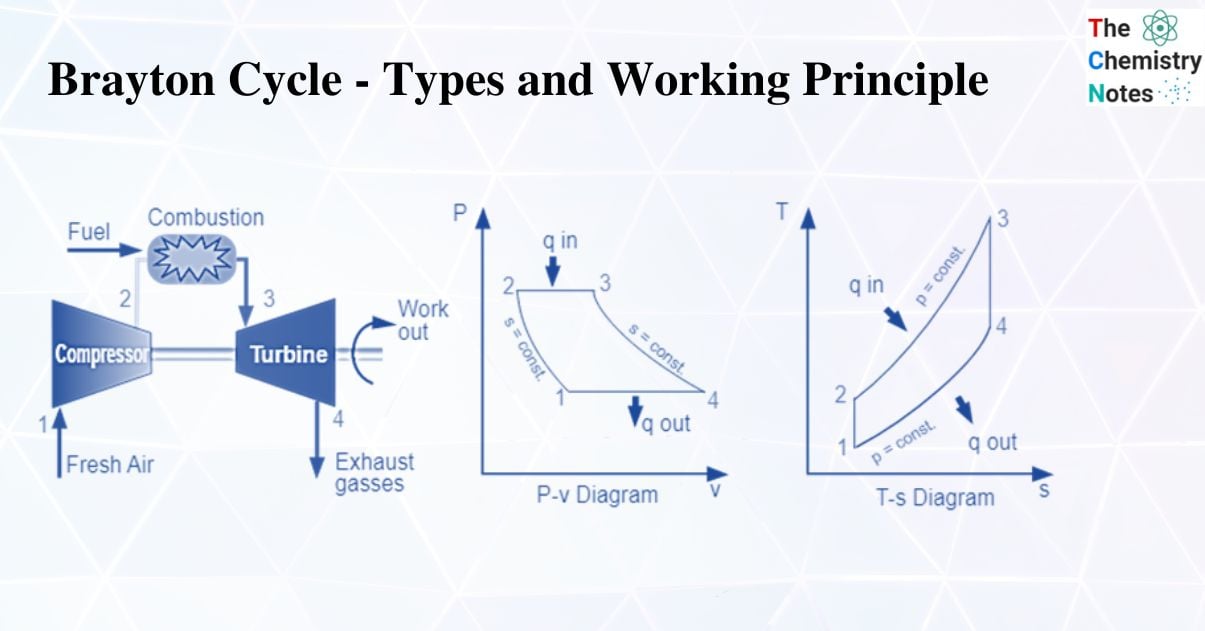
Brayton Cycle – Introduction
A Brayton cycle is a thermodynamic cycle that explains how a heat engine with constant pressure works.
A heat engine uses a Brayton cycle to extract energy from the moving fuel and air to create useful work, which is then used to propel a vehicle. The Joule cycle is another name for this cycle. The regenerator works in tandem with an external heat source in the reverse joule cycle.
In particular, some jet engines and gas turbine engines use it. The cycle entails compressing outside air, combining it with fuel, and then lighting the resulting mixture, which expands and generates power.
The Brayton cycle simulates the procedures that take place in straightforward gas turbine power cycles. The fundamental thermodynamic mechanisms that characterize the Brayton cycle serve as models for thrust engines. As a continuous-combustion device, it differs from intermittent-combustion cycles in that each region of the engine performs a single thermodynamic function as opposed to all thermodynamic processes taking place in a piston-cylinder device.
History and Invention
- John Barber’s gas turbine, which was patented in 1791, was the first gas turbine to use the Brayton’s Cycle, it was developed before the Brayton Cycle was even invented. However, due to a lack of technological advancements and other factors in the late 18th century, the gas turbine lacked sufficient power to pressurize the gases and perform useful work at the same time, so it was not used.
- George Brayton, an engineer, created the first continuous ignition combustion engine, a two-stroke engine marketed as “Brayton’s Ready Motors,” using what is now known as “The Brayton Cycle” (also known as The Joule Cycle) of thermodynamic processes. A constant pressure internal combustion engine, initially powered by vaporized gas but later on liquid fuels like kerosene, was patented in 1872 . Instead of a gas turbine and a gas compressor, the original Brayton engine utilized a piston compressor and expander.
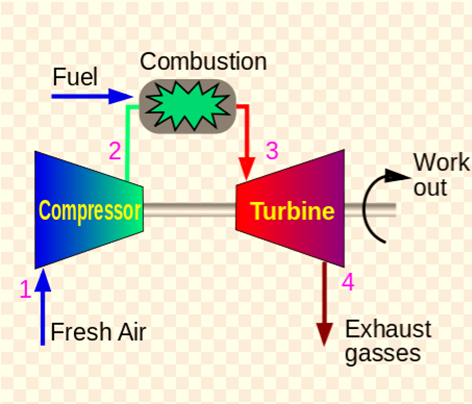
Types of Brayton Cycle
There are three distinct types of the Brayton cycle:
- Open Brayton cycle
- Closed Brayton cycle
- Reversible Brayton cycle
Open Brayton Cycle
An open Brayton cycle is a Brayton cycle in which exhaust gas is released directly into the environment.
Open Brayton cycle gas turbines with internal combustion are used in power plants. Since the majority of gas turbines (such as jet engines) are based on the open Joule Cycle with internal combustion. During this cycle, air from the surrounding atmosphere is compressed to the higher pressure and temperature of the compressor. By burning the fuel-air mixture in the airflow, the air in the combustion chamber is heated even more. Gases and combustion byproducts either expand in the turbine to pressures required by jet engines, or to pressures that are close to atmospheric pressure for engines that produce mechanical or electrical energy. The gases are released into the atmosphere directly in an open Joule Cycle.
The way that this cycle operates is as follows:
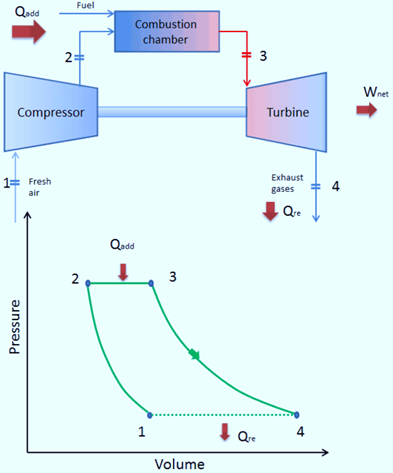
Closed Brayton Cycle
A closed Brayton cycle is one in which exhaust gases are not released into the atmosphere after passing through the turbine and being reintroduced into the compressor.
A heat exchanger is employed in this cycle to reject any extra heat. It is used in close-cycle gas turbines and high-temperature gas-cooled reactors.
In a closed Joule Cycle, the working medium (such as helium) recirculates in the loop and the compressor is refilled with the gas that was expelled from the turbine. These turbines typically employ an external combustion heat exchanger, and only a clean medium devoid of combustion byproducts passes through the power turbine. For instance, closed-cycle gas turbines and high-temperature gas-cooled reactors both employ the closed Joule Cycle.
The way that this cycle operates is as follows:
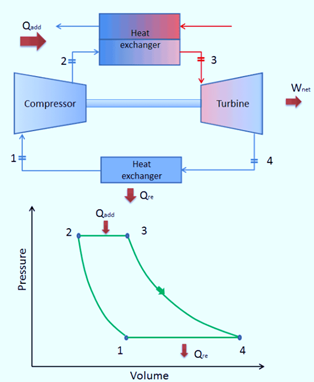
Reverse Brayton Cycle
A reverse Brayton’s cycle operates in the opposite direction. This cycle’s primary goal is to move heat from a cold reservoir to a hot reservoir rather than create work.
The second law of thermodynamics states that without an outside force acting on the body, heat cannot freely transfer from a cold body to a hot body. It means that heat can move from a cold to a hot system when an external force acts on the body or system. The same principle underlies how both heat pumps and refrigerators operate. These systems are powered by an electric motor, which requires energy from their surroundings to run.
The Reverse Brayton’s Cycle operates similarly to the regular Brayton’s cycle but runs counterclockwise through net input work. As a result, it is also known as the gas refrigeration cycle or the Bel Coleman cycle.
The air conditioning system in jet aircraft frequently uses this cycle and draws its air from the engine compressor.
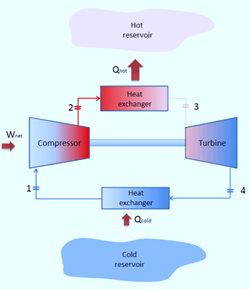
Working Principle of Brayton’s Cycle
A compressor draws in low-pressure air, which is then compressed to a higher pressure. In a combustion chamber, fuel is combined with compressed air and burned. The resultant hot gases expand as they enter the turbine. Four fundamental processes make up the Joule Cycle. They are:
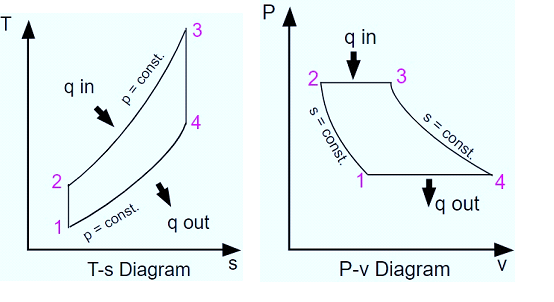
Process 1-2
The compression process is isentropic. Here, gas temperature slightly increases as a result of compression. The gas’s volume decreases because of the compression process.
Process 2-3
It is an isobaric process of heat addition. Due to the addition of heat, there is a slight volume increase. The gas’s temperature rises because it is a heat-adding process.
Process 3-4
The process of expansion is isentropic. Due to expansion, there is a small temperature drop in this area. The volume of the gas increases because it is expanding during the process.
Process 4-1
It is an isobaric process for heat rejection. Heat rejection causes a slight volume reduction. The gas’s temperature drops because it is a heat rejection process.
Thermal Efficiency
The thermal efficiency of the heat engine is calculated as the net output work (W) divided by the input heat supplied at high temperature (QH).
Brayton cycle’s formula for thermal efficiency:
nth = W / QH
To determine the Joule Cycle’s efficiency, we must first determine how much work is done in the total internal energy.
The first law of thermodynamics states that during a thermodynamic cycle, no heat is created or lost.
Internal energy = U= −w + q1 + q2 = 0
Since, in Brayton’s cycle U = 0;
w = q1 + q2
Where; q1 = Heat gained by the combustion process and q2 = Released heat after expansion
Considering gas as a perfect gas along with constant specific heat (cp):
q1 = cp (TI−TF)
q2 = cp (TF−TI)
Where, TF = The Final temperature inside the combustion chamber and TI = The initial temperature of the combustion chamber
The final equation for q1 will be if we replace the TF with T4 and the TI with T3 in accordance with the PV diagram.
q1 = cp (T4 − T3)
Brayton cycle’s thermal efficiency;
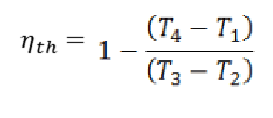
Figures source: https://www.nuclear-power.com/nuclearengineering/thermodynamics/thermodynamic-cycles/brayton-cycle-gas-turbine-engine/
Read more: Thermodynamics and Laws of Thermodynamics
References
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/brayton-cycle#
- https://web.mit.edu/16.unified/www/SPRING/propulsion/notes/node27.html
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/Thermodynamic_Cycles/Brayton_Cycle
