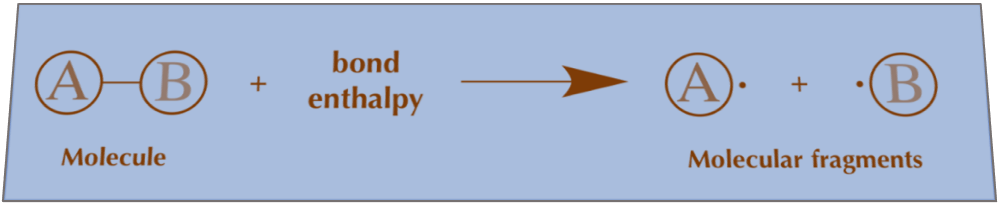
Bond enthalpy, also referred as (bond energy, bond-dissociation enthalpy, average bond energy, or bond strength) is a quantity that provides information about the strength and, by extension, the stability of a chemical bond.
The environment has an impact on bond enthalpies; the same type of bond may have different bond energies in various environments. The mean bond energy, which is an average across various molecules, helps to calculate enthalpy values.
The overall amount of energy needed to break 1 mole of a chemical bond is known as the bond enthalpy.
What is Bond Enthalpy?
The energy needed to disintegrate a chemical bond is known as the bond enthalpy. It is typically measured at 298 K and expressed in units of kJ mol-1. The molecular environment in which a given chemical bond determines the exact bond enthalpy of that bond.
Bond forming and bond breaking are two aspects of chemical reactions. Bond breaking is an endothermic process that requires energy. Whereas bond formation is an exothermic process that releases energy.
The average bond enthalpies in an energy cycle can be used to calculate the enthalpy change. Enthalpy needed to break bonds is equal to (bond enthalpies in reactants)
Hence, Enthalpy required to break bonds = ∑(bond enthalpies in reactants)
Enthalpy required to make bonds = ∑(bond enthalpies in products)
Therefore:
ΔH = ∑(bond enthalpies in reactants) – ∑(bond enthalpies in products)
ΔH is the Change in Enthalpy.
- The reaction is endothermic if more energy is released when new bonds are formed than is required to break existing bonds.
- The reaction is exothermic if more energy is released when new bonds are formed than is needed to break them.
Calculating Enthalpy Change using Bond Enthalpies
Bond enthalpies are used to calculate the enthalpy of reaction. We can use the following process to accomplish this:
In Step 1: Determine which bonds in the reactants will break and the bond enthalpies of those bonds.
Step 2: Add the bond enthalpy values for the broken bonds.
Step 3: List the new bonds that form in the products along with their negative bond enthalpies. To determine the energy released when the bond forms, keep in mind that we must reverse the sign of the bond enthalpy values.
Step 4: Add up the bond enthalpy values for the bonds formed in the produced molecules.
Step 5: To determine the enthalpy of a reaction, add the total values for bond-forming and bond-breaking (Steps 2 and 4) together.
Let us take this example into consideration:
Cl2 (g) + H2 (g) = 2HCl (g)
The preceding reaction can also be expressed as:
Cl-Cl (g) + H-H (g) = 2 H-Cl (g)
- We could deduce from this reaction that 1 H2 molecule (or 1 mole of H-H bonds) and 1 Cl2 molecule (or 1 mole of Cl-Cl bonds) must be broken to produce 2 molecules of HCl (or 2 moles of H-Cl bonds).
- The following are the bond energies:
| Bond | Bond Energy (KJ/mol) |
| H-H | 436 |
| Cl-Cl | 243 |
| H-Cl | 432 |
Using the formula and the bond energy values, we are able to determine the enthalpy change of this reaction.
ΔH = ∑(bond enthalpies in reactants) – ∑(bond enthalpies in products)
= [436 + 243] – [432 + 432]
= -185kJ
As a result, we obtain a negative H value, indicating the release of extra energy as heat. The reaction is exothermic as a result.
The environment is used to absorb energy if the calculated H is positive, indicating a lack of energy. This reaction is therefore endothermic.
Bond Energies of some important bonds in chemical reactions
| Bond | Bond Energy (KJ/mol) |
| H-H | 436 |
| H-C | 415 |
| H-O | 464 |
| H-N | 390 |
| H-Cl | 432 |
| C-C | 345 |
| N-N | 418 |
| Si-Si | 230 |
| P-Cl | 330 |
| N-O | 200 |
| H-Br | 370 |
Average Bond Energy
Other atoms in the molecule can influence bond energy.
The O-H bond in ethanol connects to a carbon atom rather than another hydrogen atom, which results in a slightly different bond energy value than the O-H bond in water. The O-H bond is in a unique setting.
When we measure identical bonds in molecules with two or more types of bonds, their bond energies differ. In water, breaking the first O-H bond requires more energy than breaking the second. We use average bond energies derived from a large number of bonds of the same type but in various environments for the aforementioned reasons.
Average bond enthalpies are always positive
Since it always takes energy to break bonds, average bond enthalpies are always positive (endothermic).
In essence, the bond enthalpies of the same type of bonds in various environments are averaged. Because they are average values, data in the book could differ slightly. As a result, bond enthalpies are roughly calculated.
Typically, we must use an enthalpy cycle to calculate the bond energy value since we are unable to do so directly. Using the enthalpy changes of the atomization of carbon and hydrogen as well as the enthalpy change of combustion or methane formation, it is possible to determine the average bond energy of the C-H bond in methane.
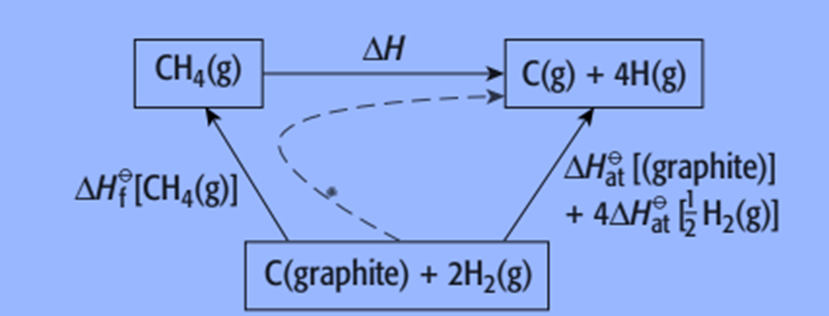
For example, the reaction for the Haber process
N2 (g) + 3H2 (g) → 2NH3(g)
The enthalpy cycle for this reaction is:
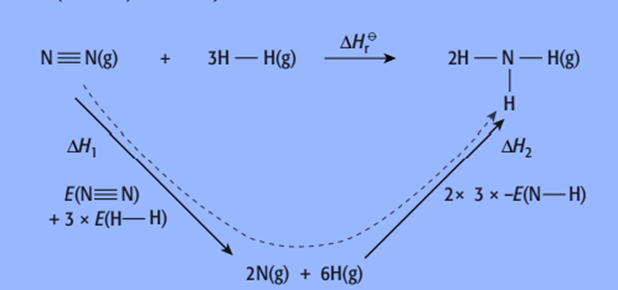
The relevant bond energies are:
(N-N) = 945 kJ/mol
(H-H) = 436 kJ/mol
(N-H) = 391 kJ/mol
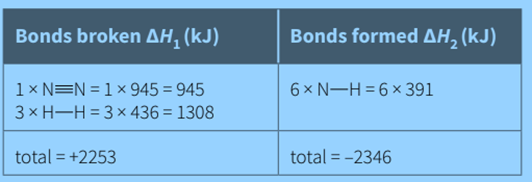
Note in these calculations that:
- one triple bond in nitrogen is broken
- three single bonds in hydrogen are broken
- Six single N-H bonds in hydrogen are formed (because each of the two ammonia molecules has three N-H bonds)
values for bond breaking are positive, as these are endothermic, and values for bond forming are negative, as these are exothermic.
From the enthalpy cycle;
ΔHr = ΔH1 + ΔH2
ΔHr = 2253 + (–2346) = –93 kJ/mol
Hence, ΔHr = (enthalpy change for bonds broken +enthalpy change for bonds formed)
Significance of Bond Enthalpies
- We can better comprehend how a chemical system uses energy during reactions.
- It describes the amount of energy required to break or form a bond and is also a measure of bond strength
- One can calculate the total change in the system’s potential energy, which is equal to ΔHrxn, by adding the bond enthalpy values for all of the bonds that are broken and formed during a reaction.
- We can determine whether a reaction will be endothermic or exothermic based on whether the enthalpy of the reaction is positive or negative.
Have a quick look in this video for the summary of the lesson. https://youtu.be/xE1gdQZcR-o
References
- Atkins, Peter and de Paula, Julio; Physical Chemistry for the Life Sciences, United States, 2006.Katherine Hurley
- https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/enthalpy-chemistry-sal/a/bond–and-enthalpy-of-reaction#
- https://www.savemyexams.co.uk/as/chemistry/cie/22/revision-notes/1-physical-chemistry/1-5-chemical-energetics/1-5-4-enthalpy- bond- energies/
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Thermodynamics/Energies_and_ Potentials/Enthalpy/ Bond_Enthalpies
- Petrucci, et al. General Chemistry Principles & Modern Applications. 9th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2007

