Cholesterol is the most abundant sterol in tissues and plasma. It is present in animal or human cells either in free or stored or combined form as cholesteryl ester. It is the fat-like waxy substance that is obtained from the liver and our diet. Animal and plant-based foods such as milk, milk products, fruits, nuts, and vegetables are good sources of cholesterol.
Interesting Science Videos
Transport of cholesterol
Lipoproteins transport cholesterol through the bloodstream. There are two types of lipoprotein; they are as follows:
HDL cholesterol: It is high-density lipoprotein. A high level of HDL protects against heart attack and stroke, it is considered good cholesterol.
LDL cholesterol: It is low-density lipoprotein. LDL can also cause an increased risk of coronary heart disease and stroke. So, it is considered bad cholesterol.
Biosynthesis of cholesterol
Cholesterol is a major component of animal cell plasma membranes. The majority of cholesterol is produced by endogenous biosynthesis, which occurs in the liver, intestine, and skin. The rest is taken up from food. Most of the cholesterol is incorporated into the lipid layer of the plasma membrane or converted into bile acids. Like most biological lipids, cholesterol is synthesized from the two-carbon acetate group of acetyl-CoA in the cytosol. The Acetyl-CoA required for this process can be obtained from a variety of sources, including -the oxidation of fatty acids, oxidation of ketogenic amino acids like leucine and lysine, and the pyruvate dehydrogenase reaction. Hence, four basic steps are involved in the synthesis of cholesterol, they are as follows:
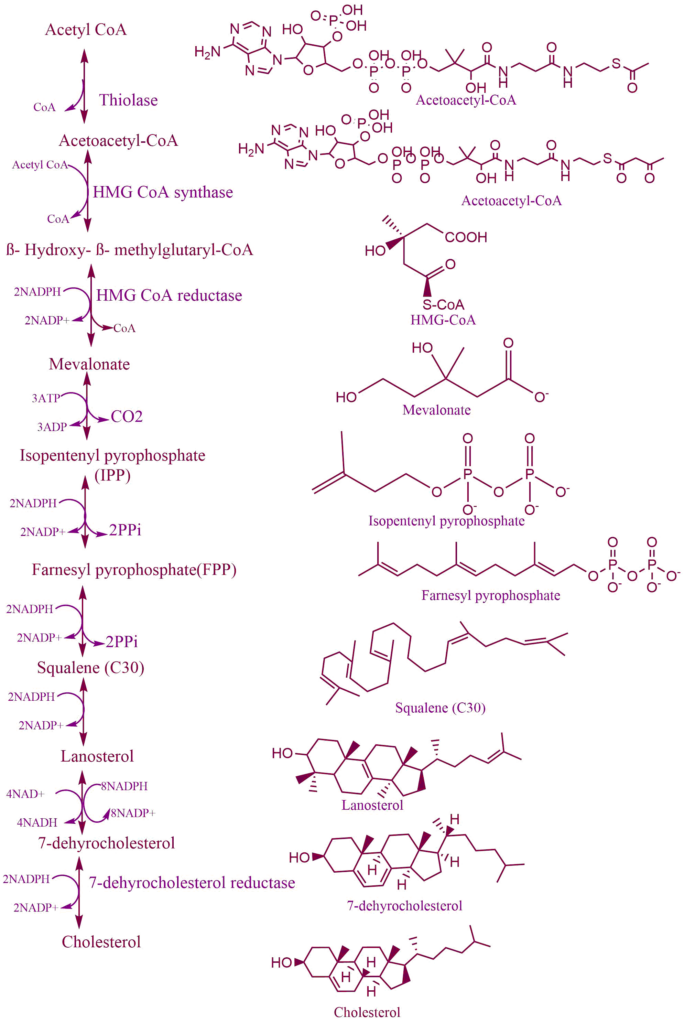
Step 1: Formation of mevalonate from acetate
The first step of cholesterol biosynthesis leads to the production of intermediate mevalonate. This step is rate liming step in cholesterol synthesis. In this step, two molecules of acetyl-CoA condense to form acetoacetyl-CoA via the action of acetoacetyl-CoA synthase. Acetoacetyl-CoA reacts with third molecules of acetyl-CoA to form six-carbon compounds, β- Hydroxy- β- methylglutaryl-CoA i.e., HMG-CoA. Thus, this reaction is catalyzed by the enzyme HMG-CoA synthase encoded by the HMGCS1 gene.
Step 2: Conversion of mevalonate into isopentyl pyrophosphate
Mevalonate is activated by two successive phosphorylations catalyzed by the enzyme’s mevalonate kinase and phosphomevalonate kinase, to yield mevalonate-5-phosphate and then mevalonate-5 diphosphate. After the phosphorylation, mevalonate undergoes decarboxylation to form C5 isoprenoid compounds i.e., isopentenyl pyrophosphate.
Step 3: Polymerization of isopentenyl pyrophosphate to form linear structure i.e., Squalene
Isopentenyl pyrophosphate undergoes isomerization to form the dimethylallyl diphosphate. The two C5 molecules condensed to yield geranyl pyrophosphate and the addition of another isopentenyl pyrophosphate produced farnesyl pyrophosphate. This can undergo dimerization in a head-to-head manner to give Squalene.
Step 4: Cyclization of squalene to form the steroid nucleus
Squalene undergoes a two-step cyclization to yield lanosterol. During the cyclization enzyme squalene epoxidase introduce molecular oxygen as an epoxide at the 2,3 positions of squalene forming the intermediate, 2,3-oxidosqualene. After that, the epoxide intermediate is converted to lanosterol. The enzyme lanosterol synthase catalyzes this step of the reaction. Thus, formed lanosterol. Subsequent removal of a methyl group on C14 and C4 and movement of double bond C8-C9 to C5-C6 from lanosterol thus produces the desmosterol. Finally, the double bond of the side chain is reduced producing cholesterol.
Regulaion of cholestrol biosynthesis
Transcriptional control
One of the sterol-regulatory element-binding proteins regulates the rate of synthesis of HMG-CoA reductase messenger RNA (mRNA) (SREBPs). SREBP is anchored to the endoplasmic reticulum in its inactive state.
When the cholesterol level falls the protein is released. Hence, to enhance transcription, the released protein migrates to the nucleus and binds the SRE of the HMG-CoA reductase gene. When cholesterol levels rise, the SREBP proteolytic release is inhibited, and the SREBP in the nucleus is rapidly degraded.
Competitive inhibition
Stations like lovastatin, mevastatin, and atrova statin are reversible competitive inhibitors of HMG-CoA reductase which are used to decrease plasma cholesterol levels in patients with hypercholesterolemia.
Feedback inhibition
HMG-CoA reductase is inhibited by the mevalonate and cholesterol also.
Proteolytic degradation of HMG-CoA reductase
HMG-CoA reductase membrane domains contain sterol-sensing regions. When cholesterol (or its derivatives) levels rise in the cell, the oligomerization state of the membrane domain of HMG-CoA reductase changes, making the enzyme more susceptible to proteolysis. This, in turn, reduces the enzyme’s activity.
Regulation by covalent modification
Phosphorylation can affect the activity of HMG-CoA reductase since the phosphorylation decreases the activity of the reductase. High glucagon levels increase the phosphorylation of enzyme thereby inactivating it, whereas elevated insulin favors the formation of the active form of HMG-CoA reductase and results in an increased rate of cholesterol synthesis. Cholesterol synthesis is also reduced at low ATP levels.
Functions of cholesterol
1. It is a major constituent of the plasma membrane and plasma lipoproteins.
2. It also enables the body to make a few hormones.
3. It is a precursor of bile salts.
4. It also facilitates the body to produce vitamin D.
5. It is also required for nerve transmission.
Effect of high cholesterol level on the body
1. Excess levels of cholesterol in the blood can block arteries to the brain increasing the risk of brain stroke.
2. High levels of cholesterol can cause chest pain, jaw pain, and stomach pain.
3. It reduces blood flow.
4. High cholesterol has also been linked to mental impairment and dementia.
References
- Satyanarayana U. & Chakrapani U. (2013). Biochemistry (4th ed.). Elsevier Health Sciences APAC. Retrieved September 9 2022 from
- https://byjus.com/biology/cholesterol/
- https://med.libretexts.org/Bookshelves/Basic_Science/Cell_Biology_Genetics_and_Biochemistry_for_Pre-Clinical_Students/06%3A_Lipoprotein_Metabolism_and_Cholesterol_Synthesis/6.01%3A_Cholesterol_Synthesis
- https://www.slideshare.net/namarta28/cholesterol-synthesis-steps-and-regulation
- https://www.hsph.harvard.edu/nutritionsource/what-should-you-eat/fats-and-cholesterol/cholesterol/
- https://themedicalbiochemistrypage.org/cholesterol-synthesis-metabolism-and-regulation/
- https://www.healthline.com/health/cholesterol/effects-on-body
- https://shcc.ufl.edu/files/2011/09/gf-cholesterol.pdf
