The bimolecular nucleophilic substitution reaction (SN2 reaction) is a one-step reaction that involves the transition state. The attack of a nucleophile on the substrate and the departure of the leaving group occur simultaneously in this reaction. Since both the substrate and the nucleophile are present in the transition state, the reaction is bimolecular.
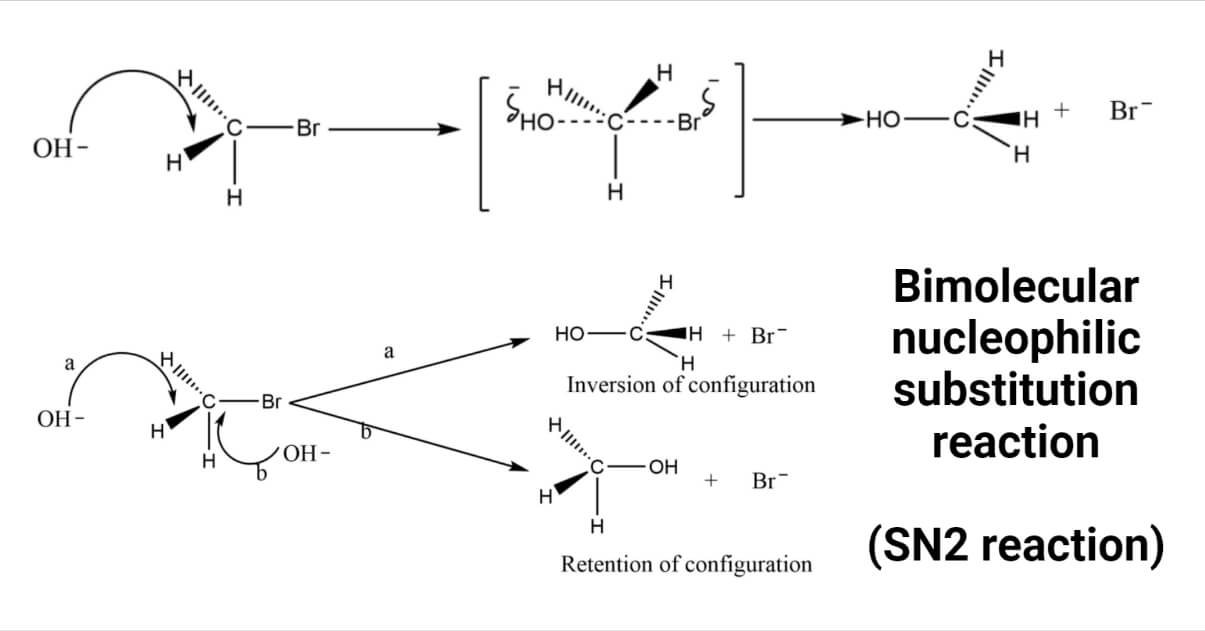
It is the reaction in which both the substrate and nucleophile are present in the slow or rate-determining step of the reaction. So, the rate of the reaction depends on the concentration of both the substrate and nucleophile. If the concentration of any one of them is doubled, then the reaction rate is also doubled. The reaction is of second-order kinetics.
Example: Let’s consider the hydrolysis reaction of methyl bromide to methyl alcohol using aq.NaOH, in which the hydroxide ion replaces the bromide ion.
Interesting Science Videos
SN2 Reaction Mechanism
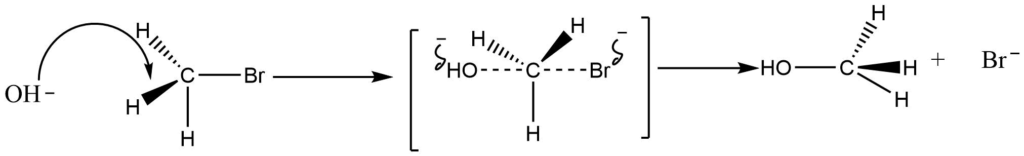
Rate ∝ [CH3Br] [OH–]
Rate = K [CH3Br] [OH–]
The hydroxide ion (nucleophile) attacks the carbon from the opposite side to that of bromine (leaving group) to experience the minimum repulsion. As the reaction proceeds through the transition state, a bond between carbon and the hydroxide ion forms, and the bond between carbon and bromine breaks. The energy necessary to break the C-Br is partly obtained from the energy released during the formation of the C-OH bond.
Stereochemistry of SN2 reaction
The backside attack of the nucleophile in the SN2 reaction mechanism causes a tetrahedral arrangement of the substrate to assume the transition state, forcing the three atoms or groups attached to carbon to assume a planar configuration. So, the hybridization of the carbon atom changes to sp2 hybridization, and the T.S. complex molecule becomes planar. The final product has an inversion of configuration, resulting in the formation of another sp3-hybridized carbon-containing, tetrahedral CH3OH molecule.
Features of SN2 reaction
- Inversion of configuration is obtained (Walden inversion).
- In the SN2 mechanisms, the rate of reaction for haloalkanes is methyl > primary > secondary >> tertiary. Due to steric hindrance, the addition of alkyl groups to the carbon atom of the carbon-halogen bond protects the carbon atom from attack by nucleophiles in the direction required for the transition state. Furthermore, 20 and 30 haloalkanes are highly polarized, and carbocation ions are formed during the reaction. As a result, their reactivity is low.
- The reaction is a concerted step reaction so, there is no possibility of the formation of a rearranged product being formed by this mechanism.
Factors affecting SN2 reaction
Nature of substrate
The transition state of the SN2 reaction has five groups surrounding the nucleophilic center i.e., the nucleophile, the leaving group, and three substituents. If all three carbon substituents were hydrogen, the incoming nucleophile and electrophilic center would have little steric repulsion. However, if one of the hydrogens is replaced with an R group (methyl or ethyl group), the steric repulsion with an incoming nucleophile increase. As a result, reactivity decreases.
Nature of nucleophile
For an SN2 reaction, nucleophiles are involved in the rate-determining step, and the nature of nucleophiles affects the rate of reaction. Generally. strong nucleophiles help to speed up SN2 reactions.
Nucleophilicity decreases across a period. For example: NH3 > H2O; RNH2 > ROH
More electronegative elements tightly hold their electrons. Due to this reason, they are less likely to donate an electron to form a new bond. As a result, they are less nucleophilic than others. For example, thiols (R-NH2) are more nucleophilic than alcohols (R-OH) because oxygen has a higher electronegativity than nitrogen.
A nucleophile with a negative charge is always stronger than its neutral counterpart. For instance, OH–> H2O; RO–> ROH. So alkoxide ions (RO–) are more nucleophilic than alcohol (ROH)
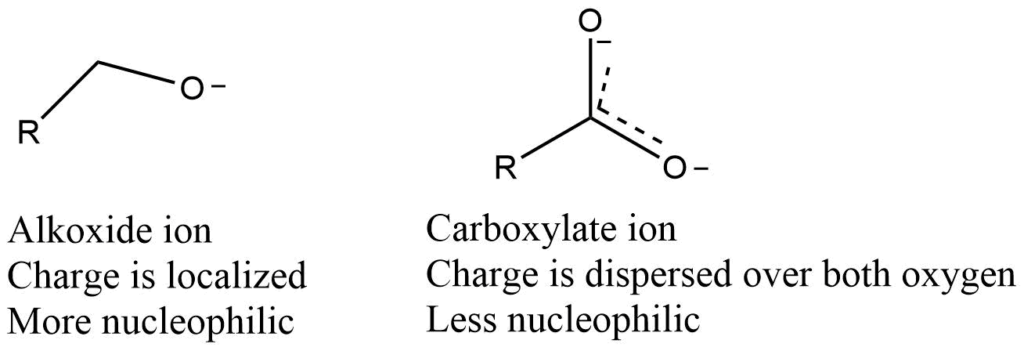
Resonating structure of nucleophile
When an electron lone pair on a heteroatom is delocalized by resonance, it becomes less reactive as well as less nucleophilic and less basic. Even though the nucleophilic atom in both cases is a negatively charged oxygen, an alkoxide ion is more nucleophilic and basic than a carboxylate group.
Leaving group
In the transition state of SN2 reactions, the bond between the carbon and the leaving group is broken. The weaker the bond between carbon and leaving group, the lower the activation energy and the faster the reaction. As a result, the reactivity order of alkyl halides is as follows.
R-I > R-Br > R-Cl > R-F
Stability of the leaving group
When the C-X bond breaks in nucleophilic substitution, the pair of electrons in the bond goes with the leaving group. As the group’s size grows, basicity decreases, and the stability of leaving the group increases. Fluoride is the worst leaving group when halogens are evaluated as leaving groups due to its small size and high charge density. The electron density decreases and the stability increases as one moves down the group. As a result, iodide is regarded as an excellent leaving group. As the group’s size grows, basicity decreases, and the stability of leaving the group grows.
Nature of solvent
The use of protic solvents (those with hydrogen-bond donating capability, such as water or alcohol) reduces the nucleophile’s power through strong solvation. Polar aprotic solvents generally favor the SN2 reactions.
References
- March, J. (1992). Advanced organic chemistry: Reactions, mechanisms, and structure. New York: John Wiley & Sons.
- Peter sykees, A guide Book to mechaism in organic chemistry, (6th edition) pearson.
- https://byjus.com/chemistry/sn2-reaction-mechanism/r.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Substitution_Reactions/SN2
- https://kpu.pressbooks.pub/organicchemistry/chapter/7-2/
- https://chem.libretexts.org/Courses/Brevard_College/CHE_202%3A_Organic_Chemistry_II/04%3A_Substitution_and_Elimination_reactions/4.05%3A_Factors_affecting_the_SN2_Reaction
- https://courses.lumenlearning.com/suny-potsdam-organicchemistry/chapter/8-3-factors-affecting-rate-of-nucleophilic-substitution-reactions/
- https://kpu.pressbooks.pub/organicchemistry/chapter/7-3-other-factors-that-affect-sn2-reactions/
