Benzaldehyde is an aromatic aldehyde in which the -CHO group is directly bonded to the aromatic ring. It is a compound with a molecular formula C7H6O that has several industrial applications, including the preparation of dyes, cosmetic products, and flavoring agents. It is also known as the oil of bitter almonds, as it is found in the glucoside amygdalin, which occurs in bitter almonds.

Preparation of Benzaldehyde
Gattermann -Koch aldehyde synthesis
In the presence of anhydrous aluminium chloride and traces of copper (I) chloride, benzene reacts with carbon monoxide and hydrochloric acid to produce benzaldehyde. This reaction is known as Gattermann -Koch reaction. Here copper (I) chloride acts as a co-catalyst.
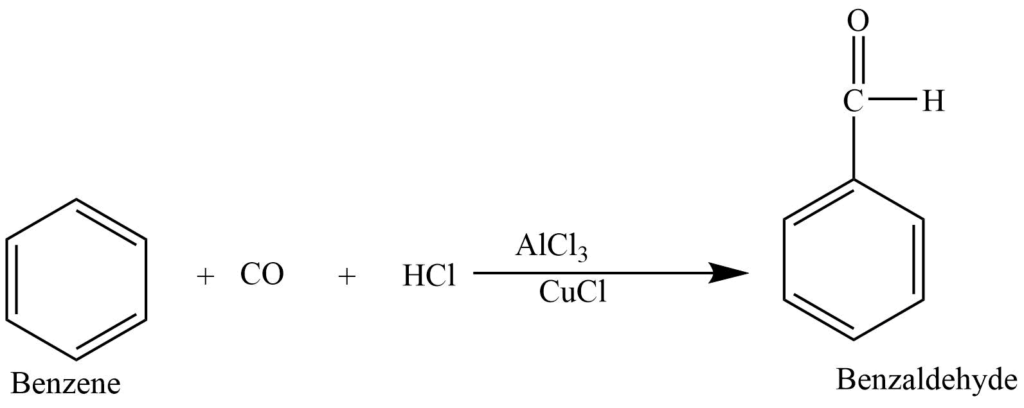
Gattermann reaction
Benzene reacts with a mixture of hydrogen cyanide and hydrogen chloride in presence of aluminium chloride to produce benzaldehyde.
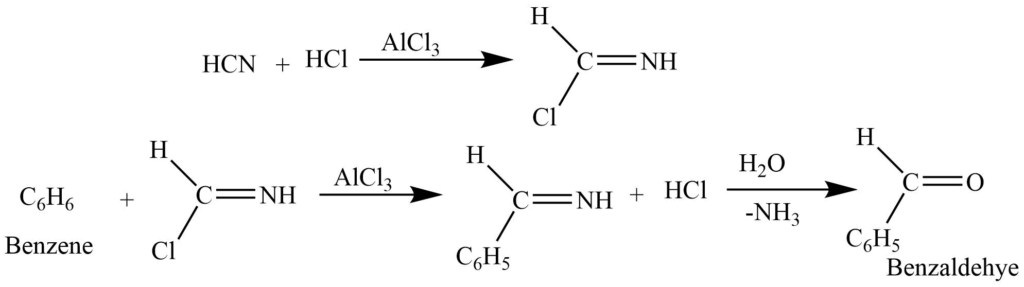
Grignard reaction
Phenyl magnesium bromide reacts with the ethyl formate to produce benzaldehyde.
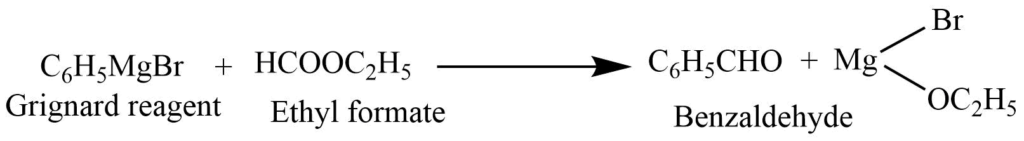
From toluene
The reaction of toluene with chromyl chloride in carbon tetrachloride solution produces an intermediate complex. Which on decomposition with water gives benzaldehyde.
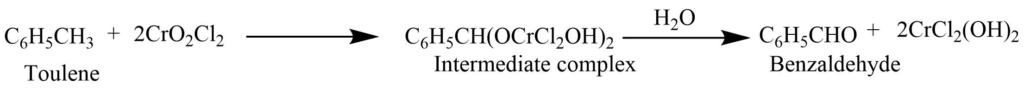
Rosenmund’s reaction
The catalytic reduction of acetyl chloride in the presence of palladium catalyst poisoned with barium sulfate gives benzaldehyde. It is a catalytic hydrogenation process of acyl chloride that results in benzaldehyde.
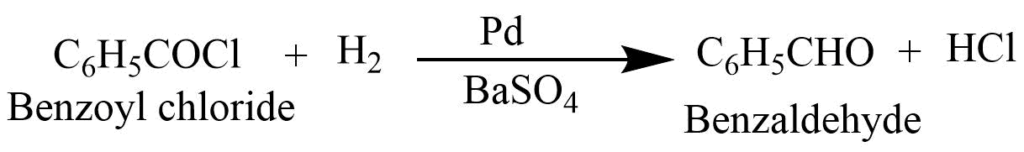
From diazonium salt
Benzene diazonium chloride on reaction with formaldoxime gives benzaldehyde.
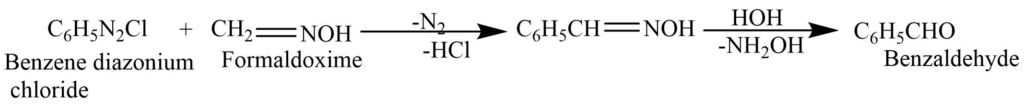
Physical properties of Benzaldehyde
- It is a colorless liquid with a Smell of bitter almonds.
- The boiling point is 179oC.
- It is readily soluble in alcohol and ether.
- Benzaldehyde is a neutral compound.
- Generally, it is stable under normal conditions.
Chemical properties of Benzaldehyde
Benzaldehyde gives the reaction of both benzene nucleus and -CHO group. Most of the reactions are similar to that of aliphatic aldehyde. However, it gives some characteristic reactions.
Reactions of -CHO group common with aliphatic aldehyde
1. it restores the pink color of Schiff’s reagent
2. Oxidation: Benzaldehyde oxidize in presence of Tollen’s reagent to form benzoic acid. Tollen’s reagent is the alkaline solution of ammoniacal silver nitrate.
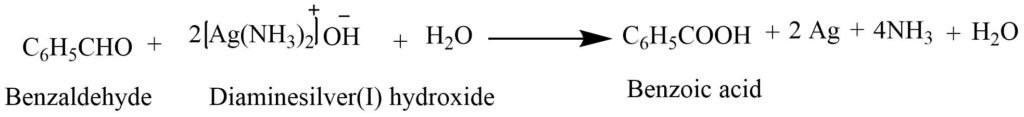
3. Reduction: Reduction of benzaldehyde with the sodium amalgam and water forms benzyl alcohol.
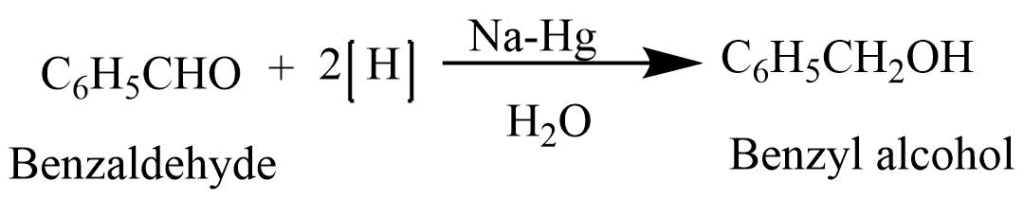
4. Benzaldehyde reacts with PCl5 to give benzal chloride.

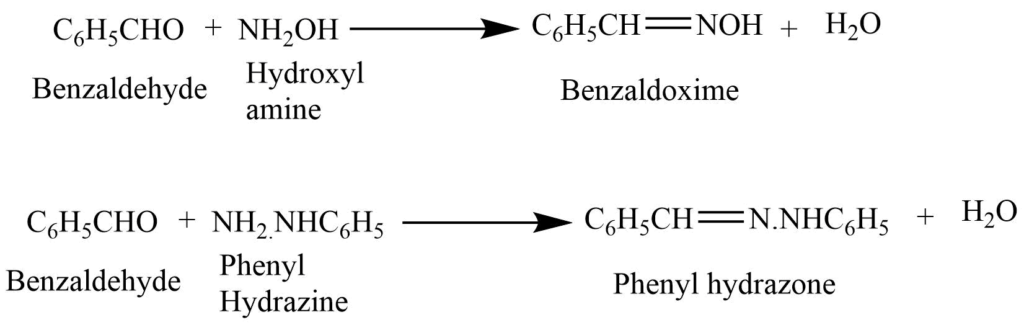
5. Reaction with hydroxyl amine and phenyl hydrazine, forms benzaldoxime and phenyl hydrazone.
Reactions of -CHO group that is not given by aliphatic aldehyde
Cannizzaro’s reaction: It undergoes disproportionation reaction in presence of strong base to give benzoic acid and benzyl alcohol. It this reaction one mole of benzaldehyde reduces to benzyl alcohol while another oxidized to give benzoic acid.
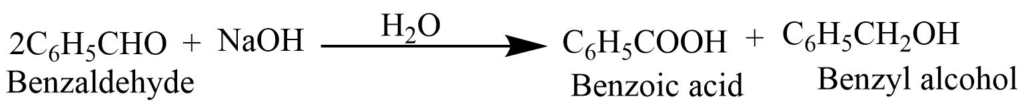
Benzoin condensation: Two moles of benzaldehyde undergo a coupling reaction on warming with KCN to form benzoin. This is called a benzoin condensation reaction.

Perkin’s reaction: Condensation reaction of benzaldehyde with an acid anhydride in presence of alkali salt of the acid gives alpha, beta-unsaturated acid i.e., Cinnamic acid. This reaction is called Perkin’s condensation reaction.
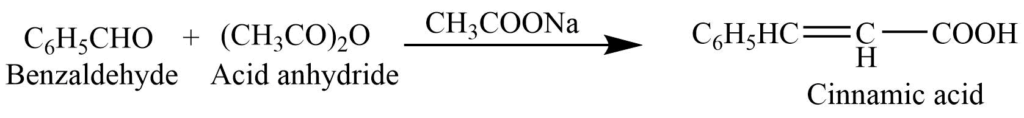
Claisen reaction: It reacts with acetaldehyde in presence of alkali to give cinnamaldehyde. This is called the Claisen condensation reaction.

Condensation of benzaldehyde with aromatic amine forms Schiff’s base.
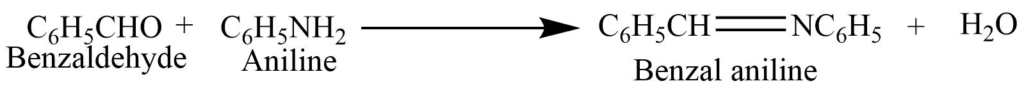
Reaction of benzene nucleus
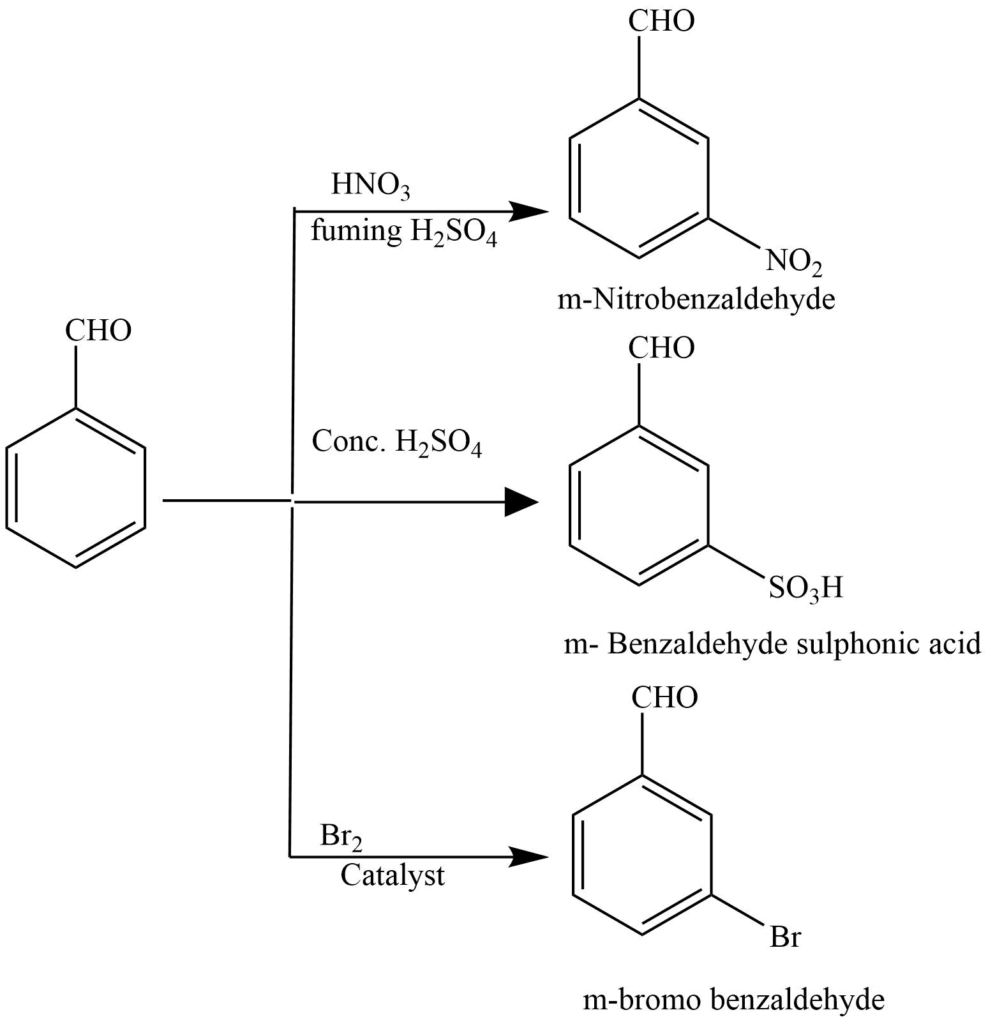
Benzaldehyde undergoes nitration, sulphonation, and halogenation reactions to give meta-substituted products.
Uses of Benzaldehyde
- It is used as a flavoring agent.
- Benzaldehyde is used to make cosmetic and personal care products.
- It is used as a solvent for oils and resins.
- Cinnamic acid obtained from benzaldehyde is used in synthetic ingo and pharmaceuticals.
- Its odor is used as a bee repellent to extract honey.
- It is used as pressure for the synthesis of chemical additives, pharmaceutical products, and plastics.
References
- https://www.vedantu.com/chemistry/benzaldehyde
- https://byjus.com/chemistry/aldehyde-group/
- https://chemicalnote.com/aromatic-aldehydes-and-ketones-preparation-and-properties/
- https://www.pharmaguideline.com/2021/12/qualitative-tests-structure-and-uses-of-benzaldehyde.html
- https://chemistrypage.in/benzaldehyde-formula-and-structure/
- https://byjus.com/chemistry/gattermann-koch-reaction-mechanism/
- https://byjus.com/chemistry/benzoin-condensation/
- https://www.britannica.com/science/benzaldehyde
- https://collegedunia.com/exams/benzaldehyde-structure-properties-uses-and-sample-questions-articleid-5390
