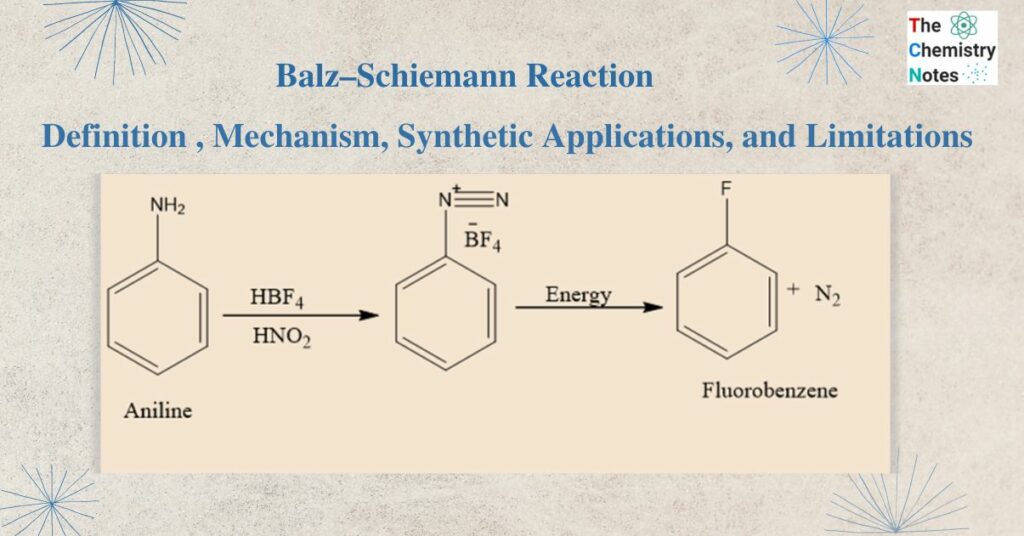
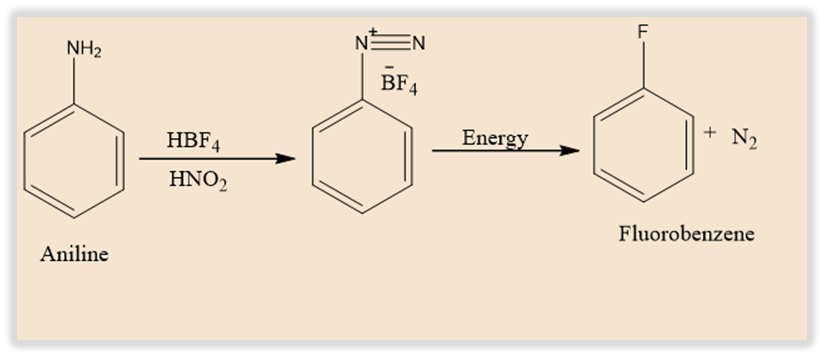
Balz–Schiemann reaction involves the transformation of a primary aromatic amine into an aryl fluoride via a diazonium tetrafluoroborate intermediate. The process is comparable to the Sandmeyer reaction, which also produces aryl halides from diazonium salts. In this process, arylamines are converted to aryl fluorides via diazotization.
The reaction is named after the German chemists Günther Schiemann and Günther Balz, who first described this reaction in 1927
The Balz Schiemann reaction uses fluorobenzene, nitrous acid, and aromatic amines as its reactants.The diazotization of aniline in the presence of HBF4 yields aryl- and heteroaryl-diazonium tetrafluoroborate salts. They are distinctive in that they are frequently stable enough to isolate. When these salts are thermally decomposed at high temperatures, fluoro-dediazoniation occurs, resulting in the creation of the corresponding aryl or heteroaryl fluoride. Typically, an inert solvent is used to conduct the salt decomposition. It would be expected that, the solvent also plays a part in controlling the process.
Interesting Science Videos
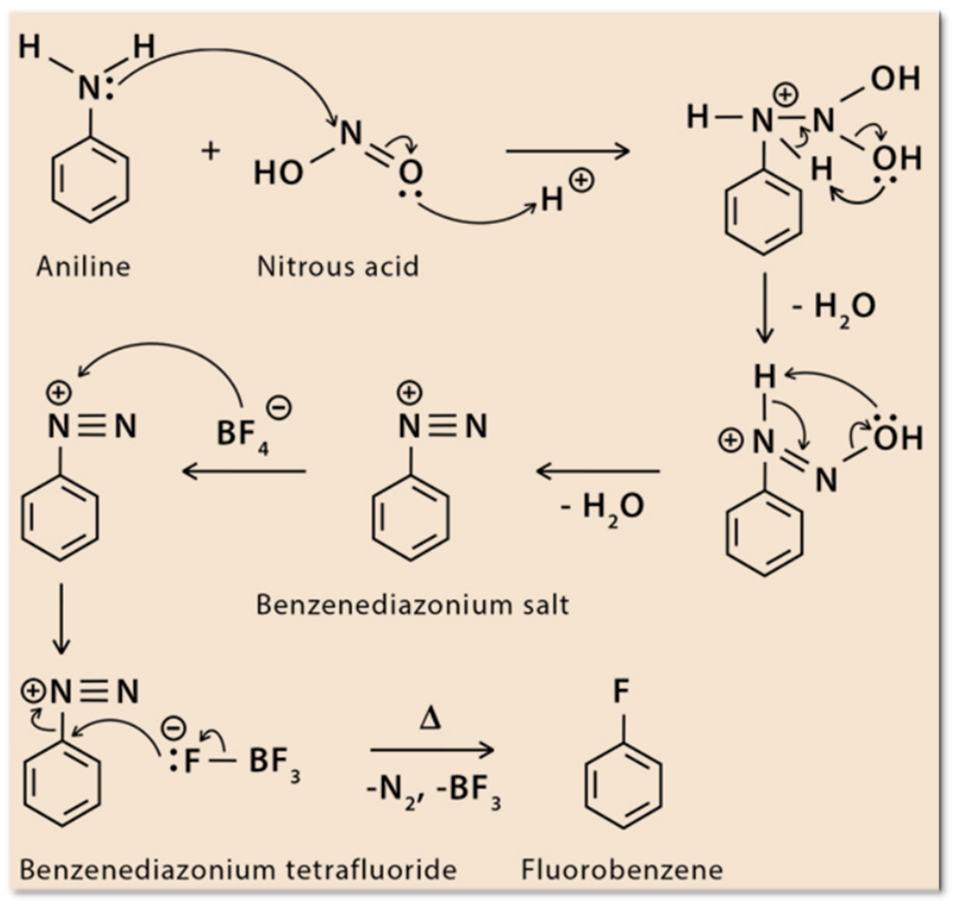
Reaction Mechanism of Balz–Schiemann Reaction
Although reactivity trends have proven unpredictable, the Balz-Schiemann reaction is thought to operate via an SN1 reaction mechanism. Under the presence of nitrous acid, aromatic amines go through a diazotization process. After adding energy and fluoroboric acid to the aryl diazonium salt, the aryl fluoride is produced via diazonium tetrafluoroborate intermediate step.
Applications of Balz–Schiemann reaction
Due to the violent character of the reaction and the difficulties in controlling the reaction, it is difficult to prepare aryl fluorides via the direct fluorination of aromatic hydrocarbons. Thus, the Balz-Schiemann method is the preferred approach to obtain aryl fluorides. For some fluorobenzene derivatives, such as 4-fluorobenzoic acid, it is also the method of choice.
Aryl fluorides that are sterically inhibited also can be prepared by using the Balz—Schiemann reaction in simple operating conditions. The reaction’s scope often corresponds with the conditions governing the breakdown of diazonium salts.
Limitations of Balz–Schiemann reaction
Although, the Balz-Schiemann reaction is still employed to produce (hetero)aryl fluorides. It demands high temperatures and is also vulnerable to harsh conditions. Apart from it, isolation of diazonium salt could be explosive.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
