Acid vs Base- Definition, 16 Major Differences, Examples
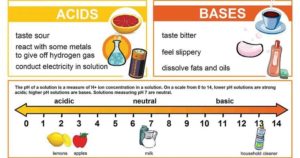
Differences between Acid and Base Image Source: Pinterest Basis Acid Base Arrhenius concept An acid is a substance that produces hydrogen ion (H+) as the only positive ion when mixed … Read more