Nitrogen is a nonmetal in Periodic Table Group V. It exists in the atmosphere as a gaseous element composed of diatomic molecules. Nitrogen makes up approximately 78 percent of the atmosphere by volume, making it the most important source of nitrogen.
Thousands of organic compounds are formed from nitrogen. The majority of known varieties are derived from ammonia, hydrogen cyanide, cyanogen, and nitrous or nitric acid. Generally, amino acids, amino acids, and amides are all derived from or closely related to ammonia.
Nitrogen gas only reacts in extremely abrasive environments because it is hard to break. In thunderstorms, for instance, the air’s nitrogen and oxygen interact. The activation energy required for this reaction to occur is provided by lightning.
N2 (g) + O2 (g) → 2NO (g) [Combination of Nitrogen and Oxygen forms Nitrogen II oxide]
2NO (g) + O2 (g) → 2NO2 (g) [The nitrogen(II) oxide formed is further oxidised by oxygen to give nitrogen(IV) oxide]
2NO2 (g) + H2O (l) +1/2 O2 (g) → 2HNO3 (aq) [Nitrogen(IV) oxide dissolves in water droplets and forms nitric acid, which rains down on the earth]
This is an important part of the natural nitrogen cycle.
What is Ammonia?
Ammonia, NH3, is a colorless gas with a pungent, irritating odor that is produced by combining large amounts of nitrogen and hydrogen.
The Haber-Bosch process is the most common commercial method of producing ammonia. Ammonia is one of the two most important nitrogen compounds in commerce, and it has a wide range of applications in the production of other important nitrogen compounds. A significant amount of commercially synthesized ammonia is converted into nitric acid and nitrates, which are nitric acid salts and esters.
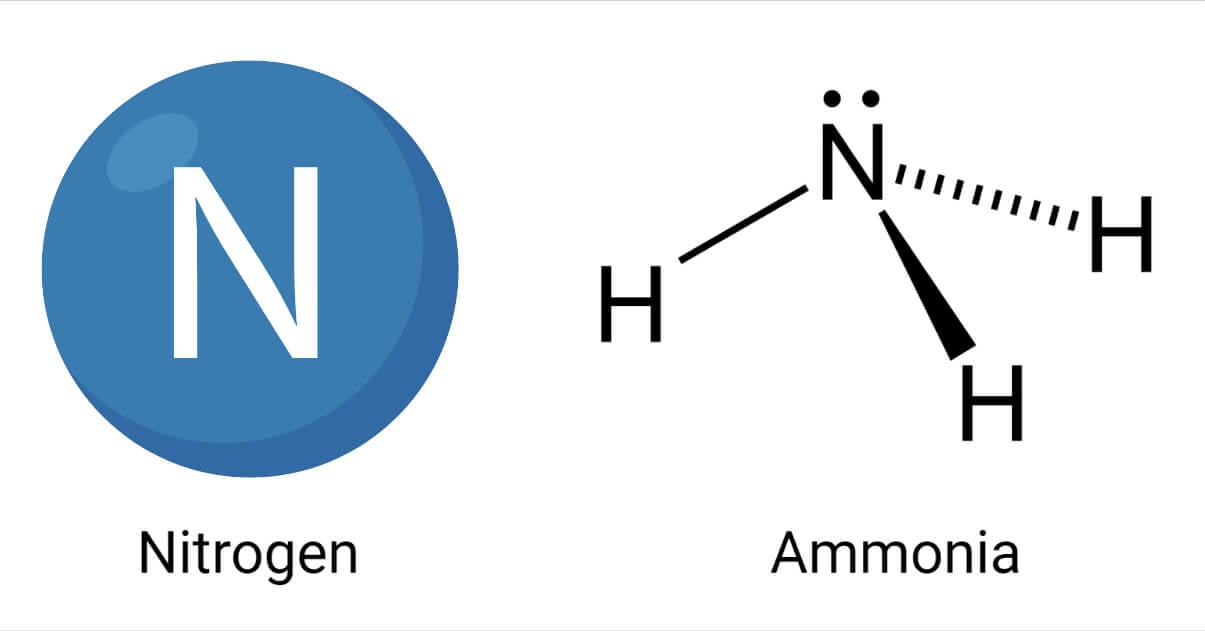
Properties of Ammonia
- Ammonia is a colorless gas with a distinctive pungent odor. It tastes bitter and burning.
- It is less dense than air.
- It is highly soluble in water and hence produces a basic solution.
- The chemical formula of ammonia is NH3.
- The repulsion between three bond pairs and one lone pair causes the pyramidal shape.
Chemical Properties of Ammonia
Using the lone pair of electrons on the nitrogen atom to accept a proton (H+) and create an ammonium ion, ammonia can function as a Bronsted-Lowry base:
NH3 (aq) + H+ (aq) → NH4+ (aq)
Ammonia dissolves readily in water. Since OH- ions are formed, the NH3 aqueous solution is a weak base.
NH3 + H20 → NH4+ + OH–
When ammonia reacts with an acid, ammonium salts are formed.
ZnS04 + 2NH4OH (g) → Zn(OH)2 + (NH4)2SO4
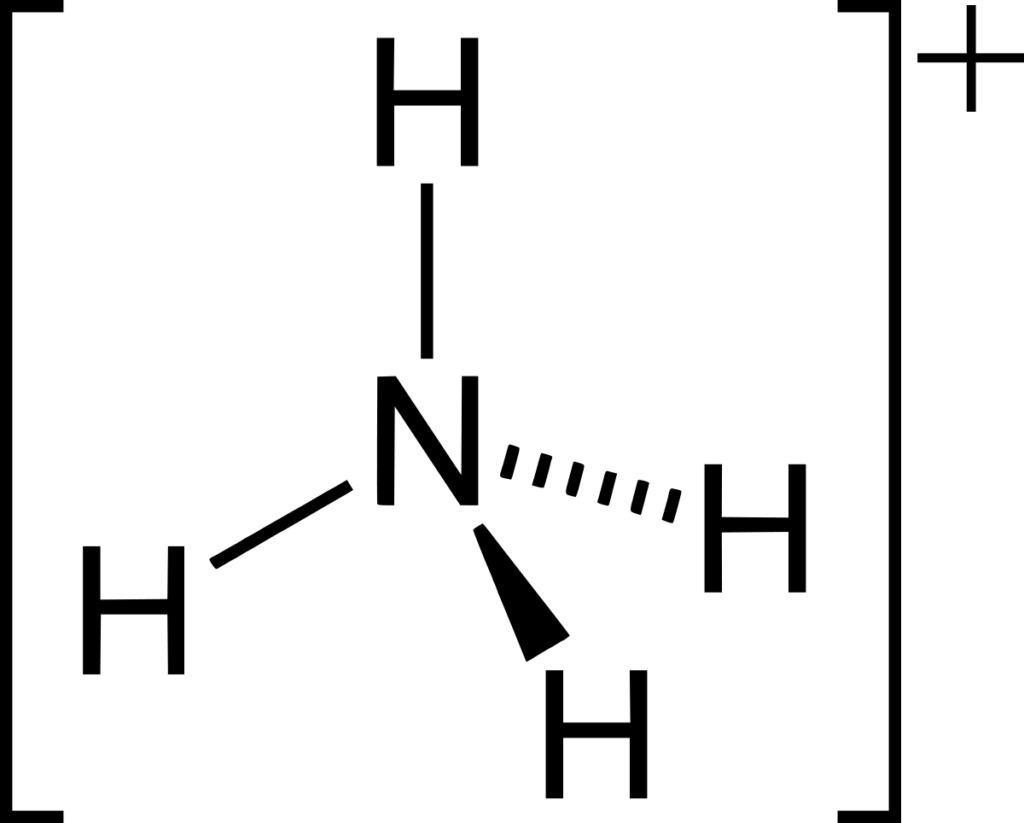
Ammonium ion: Ammonia can form a co-ordinate (dative covalent) bond with a proton using its lone pair.
Preparation of Ammonia gas from Ammonium salt
In an acid-base reaction, ammonia gas can be made from an ammonium salt and a base:
2NH4Cl (s) + Ca(OH)2 (s) → CaCl (s) + 2NH3 (g) + 2H2O (l) (In presence of heat)
Properties of equation:
- Heat is used to combine ammonium chloride (NH4Cl) and calcium hydroxide (Ca(OH)2).
- NH4+ is an acid (proton donor), and OH– is a base (proton acceptor)
- This acid-base reaction can be used to detect the presence of ammonium ions in an unknown solution.
- If the unknown solution contains ammonium ions, the ammonia gas will be formed when it reacts with calcium hydroxide.
- This ammonia gas causes damp red litmus paper to turn blue.
- As it contains no impurities, nitrogen obtained from this reaction is less dense than nitrogen isolated from air.
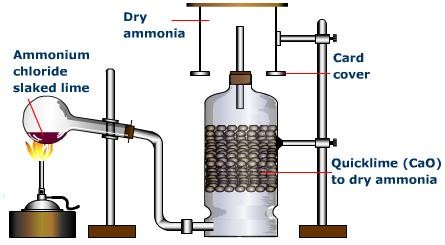
Precaution: The round-bottom flask is mounted with its neck slanted downward (the flask is slanted) to prevent condensation from trickling back into the hot part of the flask and cracking it.
Note: This reaction is also known as the displacement of ammonia.
CaO, or calcium oxide, is used as a drying agent. Calcium chloride, CaCl2, and sulfuric acid, H2SO4, are not used as drying agents because they react with ammonia.
H2SO4( aq) + 2NH3 (g) → (NH4)2SO4 (aq)
CaCl2 (s) + 4NH3 (g) → CaCl2. 4NH3 (s)
Industrial Preparation of Ammonia
The Haber process
Haber process, which is based on the direct combination of the gases, is used in industry to produce ammonia from nitrogen and hydrogen.
Raw Materials:
- Nitrogen [from the fractional distillation of liquid air].
- Hydrogen [from the electrolysis of brine or from steam reforming].
Process: Nitrogen and hydrogen are mixed in a volume ratio of one to three, purified, and dried by passing the mixture through concentrated sulfuric acid.
The mixture is then passed through a finely divided iron catalyst in a catalyst chamber that has been preheated to 450°C -500°C. The gases in this chamber are kept at a pressure of about 200 atm. The gases combine reversibly to produce ammonia under these conditions.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
The ammonia gas is liquefied and stored after being cooled in a freezing compartment. Unreacted nitrogen and hydrogen are recycled back into the catalyst chamber.
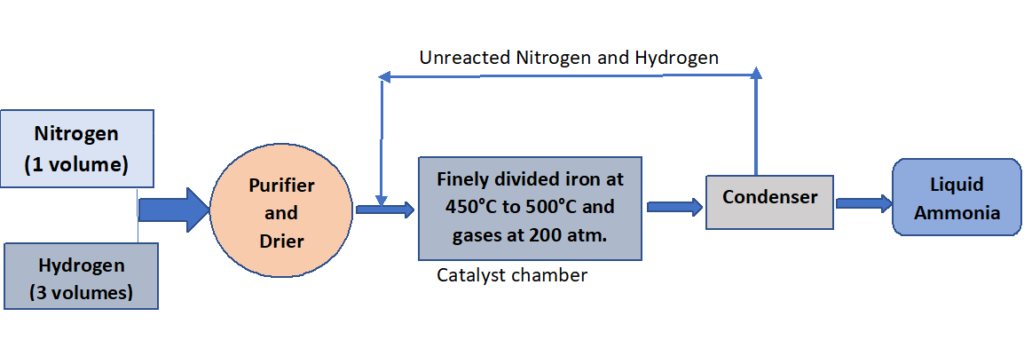
Required conditions for maximum yield:
- (400 – 450) °C.
- 200 atm (equivalent to 20000 kPa).
- Use of fine iron as a catalyst.
The equation requires that nitrogen and oxygen gas be fed into the reactor in a 1:3 ratio. Excess reactants are discarded because they take up space in the reactor and reduce the efficiency of the catalyst because they have nothing to react with.
The production of ammonia is a balanced exothermic reaction. Maintain a low temperature, according to Le Chatelier’s principle, to shift the position of equilibrium to the right as much as possible (to increase the yield). (400 – 450)°C, on the other hand, is not a low temperature.
A low temperature will slow down the reaction, despite having a high yield. It will take a long time to complete the reaction, and it is not economically feasible.
As a result, (400 – 450)°C is the compromise temperature that produces a sufficient yield in a short period.
According to Le Chatelier’s principle, as pressure increases, the position of equilibrium shifts to the right because there are fewer molecules on the right side of the equation. Furthermore, high pressure can accelerate the rate of reaction. As a result, the requirement of 200 atm pressure.
Higher pressures are not applicable because: it is expensive to build and maintain the pressure-resistant pipes and generators, which raises production costs; and there is a risk of the pipes exploding.
To speed up the reaction, use a fine iron catalyst Although it has no effect on the equilibrium position, it is necessary because the reaction would take too long to complete without it.
Under these conditions, approximately 15% of the nitrogen and hydrogen convert to ammonia. Unreacted molecules are recycled again, resulting in an overall conversion rate of about 98 percent.
Test for Ammonia gas
Insert moist red litmus paper into the unknown gas gas jar. If the unknown gas has a choking odor and turns the litmus paper blue, it is ammonia.
A glass rod is submerged in concentrated hydrochloric acid before being lowered into a gas jar containing an unknown gas. If the gas is ammonia, it will produce dense white fumes of ammonium chloride.
Uses of Ammonia
- As a fertilizer. Common fertilizers include ammonium sulfate, ammonium nitrate, ammonium phosphate, and urea, CO(NH2)2. Production of nitrogenous fertilizers as nitrogen is essential for plant growth.
- The production of nitric acid, HNO3, through ammonia oxidation in the Ostwald process.
- As a refrigerant, for example, in large-scale refrigeration plants like ships and warehouses.
- For Water softening.
- Getting rid of greasy stains.
- Production of hydrazine, which is used as rocket fuel.
Ammonium salts
Ammonium salts are ionic compounds that contain the ion ammonium (NH4). The cation is (+). They are prepared by neutralizing ammonia or aqueous ammonia with the appropriate acid.
NH3 (g) + HCl (aq) → NH4Cl (aq)
NH4OH (aq) + HCl (aq) → NH4Cl (aq) + H2O (l)
- All common ammonium salts are white crystalline solids.
- They dissolve readily in water and completely ionize, hence they are strong electrolytes.
- All ammonium salts decompose on heating, except ammonium chloride which sublimes.
Uses of Ammonium Salts
- In dry cells, ammonium chloride serves as an electrolyte.
- concentrated ammonium sulfate is a weed killer.
- Ammonium nitrate, for the production of explosives.
- Ammonium carbonate commonly prevents dizziness and fainting in smelling salts.
Nitrogenous Fertilizers
Nitrogenous fertilizers include ammonium sulphate and ammonium nitrate.
| Ammonium sulphate, (NH4)2SO4 | 2NH3 (g) + H2SO4 (aq) → (NH4)2SO4 (s) |
| Ammonium nitrate, NH4NO3 | NH3 (aq) + HNO3 (aq) → NH4NO3 (aq) |
| Ammonium phosphate, (NH4)3PO4 | 3NH3 (g) + H3PO4 (aq) → (NH4)3PO4 (aq) |
| Urea, (NH2)2 CO | CO2 (g)+ 2NH3 (aq) CO(NH2)2 (aq) + H2O (l) |
Go through the post for details of Nitrogen https://scienceinfo.com/nitrogen-element/
References
- Smith, D. (1990). Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry (Cambridge Texts in Chemistry and Biochemistry). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622922
- Mingos, D. M. P. Essential Trends in Inorganic Chemistry. Oxford University Press, 1998.
- Lee, J D. Concise Inorganic Chemistry. London: Blackwell Science, 2006. Print.
- Cotton, F A, and F A. Cotton. Advanced Inorganic Chemistry. , 1999. Print.
- https://astarrevision.com/cie_chemistry/13-1nitrogen-compounds/
- https://newsblaze.co.ke/nitrogen-and-its-compounds-form-3-chemistry-notes-in-pdf/
- https://byjus.com/chemistry/ammonium-chloride/
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/2-inorganic-chemistry/2-4-nitrogen–sulfur/2-4-1-nitrogen-and-its-compounds/
- https://notes.papacambridge.com/viewer/caie/cambridge-advanced-cambridge-international-as-and-a-level-subjects-chemistry-9701-as-chemistry-revision-topical-notes-1-chapter-14-nitrogen-and-sulphur-pdf
