Interesting Science Videos
Amine Definition
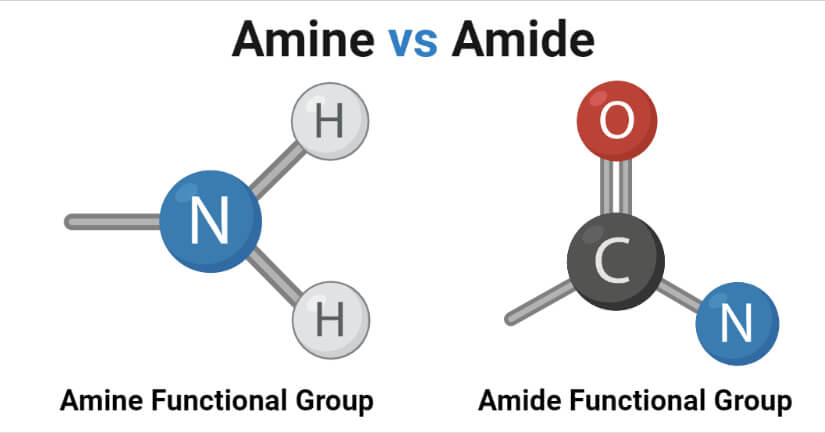
Amines are derivates of ammonia where one or more hydrogen atoms bonded to the nitrogen atom are replaced by a substituent like and alkyl or aryl group to form organic compounds.
- Amines are important organic compounds as these form amino acids and proteins, which are basic building blocks of living systems.
- Inorganic derivatives of ammonia are also referred to as amines. The substituent –NH2 found in various organic compounds is called an amino group.
- Amines can be arylamines or alkylamines depending on the presence of alkyl groups or aryl groups bonded to the amino group.
- Besides, amines can also be classified into three further categories; primary amines, secondary amines, and tertiary amines.
- Primary amines are amines where one of the three hydrogen atoms bonded to the nitrogen atom is replaced by an alkyl or aryl group.
- Secondary amines are amines where two of the three hydrogen atoms bonded to the nitrogen atom are replaced by an alkyl or aryl group.
- Tertiary amines are amines where all three hydrogen atoms bonded to the nitrogen atom are replaced by an alkyl or aryl group.
- The nomenclature of amines can be done either with a prefix ‘amino’ or a suffix ‘amine’.
- The chemical and physical properties of amines are influenced by both intermolecular and intramolecular hydrogen bonding.
- Amines exhibit basic properties due to the presence of a lone pair of electrons in the nitrogen atom.
- Different amines like aniline and ethanolamines have industrial importance in making rubber, dyes, and pharmaceutical products.
- These amines can be produced either via chemical reduction of other classes of organic nitrogen compounds or by reactions between ammonia with other organic compounds.
Amide Definition
Amides are derivatives of ammonia where one or more hydrogen atoms bonded to the nitrogen atom are replaced by the carbon atom of a carbonyl group.
- The amide group represents a peptide bond when it is present in the primary chain of proteins, and an isopeptide bond when present in the side chain.
- Amides or the amide group can be considered a derivative of a carboxylic acid where the hydroxyl group is replaced by an amine group. These are termed carboxamide.
- Amides, like amines, can be grouped as primary, secondary, or tertiary amides depending on the replacement of one, two, or three hydrogen atoms bonded to nitrogen in the amine group.
- Amides are named by adding the term ‘amide’ as a suffix to the parent acid’s name.
- The amides are neutral to slightly acidic due to the presence of a carbonyl group which reduces the basicity of the –NH2 group.
- Amides derived from ammonia are usually solids, except formaldehyde which exists in the liquid state. Amides with five or fewer carbon atoms are soluble in water, whereas the others remain insoluble.
- Amides have a higher boiling point and are good solvents for both organic and inorganic compounds.
- Amides linked together to form larger molecules are called polyamides which are found in large amounts in the protein system of living beings.
- One of the most important characteristics of amides is hydrolysis to form acids and amines. The reaction is extremely slow but can be catalyzed by a strong acid or base.
11 Key Differences (Amine vs Amide)
| Characteristics | Amine | Amide |
| Definition | Amines are derivates of ammonia where one or more hydrogen atoms bonded to the nitrogen atom are replaced by a substituent like and alkyl or aryl group to form organic compounds. | Amides are derivatives of ammonia where one or more hydrogen bonds bonded to the nitrogen atom are replaced by the carbon atom of a carbonyl group. |
| Nomenclature | Amines are named by adding the prefix ‘amino’ or the suffix ‘amine’ before and after the parent term, respectively. | Amides are named by adding the suffix ‘amide’ after the parent term. |
| Carbonyl group | Amines do not have a carbonyl group in their structure. | Amides have a carbonyl group attached to the nitrogen atom. |
| Boiling point | Amines have relatively lower boiling points. | Amides have relatively higher boiling points. |
| Classification | Amines can be classified as primary, secondary, and tertiary amines depending on the number of alkyl groups bonded to the nitrogen atom. | Amides can be classified as primary, secondary, and tertiary amides. |
| Chemical properties | Amines exhibit basic properties. | Amides exhibit acidic properties. |
| Atoms | Amines consist of carbon, hydrogen, and nitrogen atoms. | Amides consist of carbon, hydrogen, nitrogen, and oxygen atoms. |
| Solubility | Amines are soluble in water, and the solubility decreases with the increase in the number of side chains in the compound. | Only shorter amides are soluble in water. |
| Physical state | Low molecular weight amines exist in the gaseous state at room temperature. | Most amides exist in the solid-state at room temperature. |
| Production | Amines are produced by the reaction between ammonia and a hydrocarbon like an alkyl halide. The process is referred to as the alkylation of ammonia. | Amides are produced by the reaction between carboxylic acids and amines or ammonia. The process is referred to as amidation. |
| Examples | Some examples of amines include acetamide, dopamine, epinephrine, and histamine. | Some examples of amides include acetamide and paracetamol. |
Examples of amines
Dopamine
- Dopamine is a neurotransmitter that is essential in the nervous system of living beings, which is an organic compound of the catecholamine and phenethylamine families.
- A dopamine molecule consists of a catechol structure with an amino group attached via an ethyl chain.
- Dopamine is the simplest catecholamine, chemically termed a substituted phenethylamine.
- Dopamine is an organic base that is highly water-soluble and relatively stable than other similar amines.
- Dopamine is synthesized in various cell types, especially neurons and cells of the medulla in the adrenal glands.
- Dopamine is essential as it is involved in signal transduction by binding to and activating cell surface receptors. These molecules have specific receptors that are engaged in the reception and transmission of signals in neural signals.
Examples of amides
Acetamide
- Acetamide or ethanamide is an organic compound that is the simplest amide produced from acetic acid.
- The molecular formula of acetamide is CH3CONH2, resulting from the formal condensation of acetic acid and ammonia. It is often considered an intermediate between acetone and urea.
- Acetamide is primarily used as a solvent and a plasticizer. It can result in mild skin irritation from acute to chronic exposure.
- Acetamide is also essential for the production of methylamine and is used as a stabilizer and fire suppressant.
References and Sources
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 178, Acetamide” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Acetamide. Accessed 25 February, 2021.
- 2% – https://www.differencebetween.com/difference-between-amine-and-vs-amide/
- 2% – https://www.difference.wiki/primary-amines-vs-secondary-amines-vs-tertiary-amines/
- 2% – https://en.wikipedia.org/wiki/Dopamine
- 1% – https://www.coursehero.com/file/9806557/chapter16-Amines-and-Amides-1/
- 1% – https://www.coursehero.com/file/67658923/exam-4pdf/
- 1% – https://www.chegg.com/homework-help/questions-and-answers/6-methylamine-ch3nh2-important-industrial-chemical-production-various-pharmaceuticals-pest-q45335022
- 1% – https://www.britannica.com/science/amine
- 1% – https://www.britannica.com/science/amide
- 1% – https://www.askdifference.com/amide-vs-amine/
- 1% – https://vivadifferences.com/difference-between-amine-and-amide/
- 1% – https://guides.hostos.cuny.edu/che120/chapter5
- 1% – https://en.wikipedia.org/wiki/Carboxamide
- 1% – https://en.m.wikipedia.org/wiki/Amine
- 1% – https://deemagclinic.com/2017/04/14/3310/
- 1% – https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Carboxylic_Acids/Properties_of_Carboxylic_Acids/Carboxyl_Derivatives
- 1% – https://askinglot.com/what-is-the-structure-of-acetamide
- 1% – https://answers.yahoo.com/question/index?qid=20100710093318AAcXtlf
- 1% – https://answers.yahoo.com/question/index?qid=20090504063210AAhAr2n
- <1% – https://www.bioexplorer.net/building-blocks-of-proteins.html/
- <1% – https://quizlet.com/204220829/chem-test-4-flash-cards/
- <1% – https://pubchem.ncbi.nlm.nih.gov/compound/acetamide
- <1% – https://ir.library.oregonstate.edu/downloads/td96k321q
- <1% – https://en.wikipedia.org/wiki/Amides
- <1% – https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Exemplars_and_Case_Studies/Exemplars/Environmental_and_Green_chemistry/Organic_Nitrogen_Compounds%3A_Amines
