Aluminium, with the atomic symbol Al, has an atomic number of 13 and is found in Periodic Group 13. Aluminum, a post-transition metal, is a solid at ambient temperature. Aluminum is a metal, and it belongs to the third period. It possesses three valence electrons and is somewhat reactive chemically. As a result, it is not found in nature in its pure form. Aluminum will readily bond with most nonmetals.
History of Aluminium
- It has just been 160 years since the element aluminium was discovered and 100 years since a viable manufacturing process was devised, and today more aluminium is produced each year than all other nonferrous metals combined.
- Scientists recognized the presence of an unknown metal in alum as early as 1787, but they did not have a method to extract it until 1825.
- Before 5000 BCE, humans in Mesopotamia were manufacturing beautiful pottery from a clay that was mostly composed of an aluminum compound, while Egyptians and Babylonians employed aluminum compounds in various chemicals and medicines about 4,000 years ago. Pliny is referring to alumen, now known as alum, an aluminum compound that was widely used in the ancient and medieval cultures to fix dyes in textiles. Chemists such as Antoine Lavoisier recognized alumina as a potential source of a metal in the second half of the 18th century.
- Danish scientist Hans Christian Oersted was the first to manufacture trace amounts of aluminum.
- Friedrich Wöhler, a German scientist, found a new method of obtaining aluminum two years later. By 1845, he had produced big enough samples to determine some of aluminum’s basic properties.
- Henri Étienne Sainte-Claire Deville, a French chemist, refined Wöhler’s method in 1854.
- Charles Martin Hall in the United States and Paul-Louis-Toussaint Héroult in France discovered (1886) the modern method of commercially producing aluminum: electrolysis of purified alumina (Al2O3) dissolved in molten cryolite (Na3AlF6).
- During the 1960s, aluminum surpassed copper to become the world’s leading producer of nonferrous metals.
Occurrence of Aluminium
Overall, the Earth has approximately 1.59% aluminum by mass (ranking sixth in abundance by mass). Aluminum occurs in higher abundance in the Earth’s crust than in the rest of the Universe because it rapidly forms an oxide, becomes bonded into rocks, and remains in the crust, whereas less reactive metals sink to the core. Aluminium is the most prevalent metallic element in the Earth’s crust (8.23% by mass) and the third most plentiful element overall (after oxygen and silicon). Aluminium is found in a wide range of silicates in the Earth’s crust. The Earth’s mantle, on the other hand, is only 2.38% aluminum by mass.
Aluminium is practically never discovered in its elemental state due to its great affinity for oxygen; instead, it is found in oxides or silicates. Aluminum is found in igneous rocks primarily as aluminosilicates in feldspars, feldspathoids, and micas; in soil as clay; and, after further weathering, as bauxite and iron-rich laterite. The primary aluminum resource is bauxite, a combination of hydrated aluminum oxides. Crystalline aluminum oxide (emery, corundum), which occurs in a few igneous rocks, is mined as a natural abrasive or as rubies and sapphires in finer variants. Aluminium can also be found in other gemstones like topaz, garnet, and chrysoberyl. Alunite and cryolite are two commercially important aluminum minerals among many others.
The important ores of aluminium are:
- Bauxite – Al2O3.2H2O
- Corundum – Al2O3
- Cryolite – Na3AlF6
Isotopes of Aluminium
Aluminium (13Al) has 22 known isotopes ranging from 22Al to 43Al, as well as four recognized isomers. Only 27Al (stable isotope) and 26Al occur naturally, however 27Al accounts for almost all natural aluminum. Except for 26Al, all radioisotopes have half-lives of less than 7 minutes, and the majority of them have half-lives of less than a second. Aluminium isotopes are used to date marine sediments, manganese nodules, glacial ice, quartz in rock exposures, and meteorites.
Elemental Properties of Aluminum
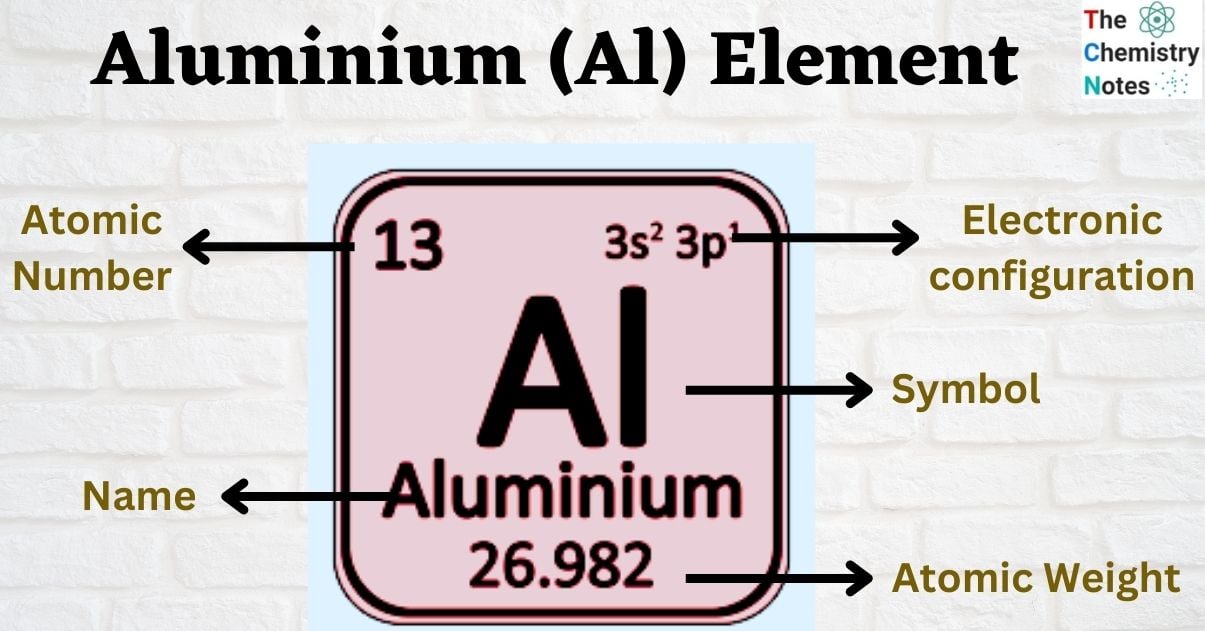
| Electronic Configuration | [Ne] 3s2 3p1 |
| Atomic Number | 13 |
| Atomic Weight | 26.982 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 13, 3, p-block |
| Density | 2.70 g.cm -3 at 20 °C |
| Ionic radius | 0.05 nm |
| Van der Waals radius | 0.143 nm |
| Electron shells | 2, 8, 3 |
| Electrons | 13 |
| Protons | 13 |
| Neutrons in most abundant isotope | 14 |
Physical Properties of Aluminum
- Commercial aluminum (99 to 99.6 percent pure) with trace levels of silicon and iron is robust and strong while pure aluminum (99 to 99.6 percent pure) is extremely soft and feeble.
- With the exception of magnesium, aluminum is the least dense of all commercial metals. Aluminum makes a great reflector, especially for UV light, when the proper surface is used.
- Silvery-white in color, aluminum has no flavor or odor.
- A face-centered cubic form can be seen in the aluminum crystal.
- Because of the development of impurity segregations, the lattice becomes concentrated in the less pure metal. Many other physical characteristics are also impacted by purity.
| Color/physical appearance | Solid, non-lustrous, silvery-white with slight bluish tint. |
| Melting point/freezing point | 660.323°C, 1220.581°F, 933.473 K |
| Boiling point | 2519°C, 4566°F, 2792 K |
| Density | 2.70 g/cm3 |
| Malleability | Yes |
| Ductility | Yes (High ductility) |
| Thermal conductivity | 237 W/(m⋅K) |
| Thermal Expansion | 23.1 µm/(m⋅K) (at 25 °C) |
Chemical Properties of Aluminum
- When aluminum is exposed to moist air, it combines with oxygen to produce aluminum oxide.
- When aluminum is in pulverized form, it is highly flammable when exposed to a flame.
- There are numerous compositions of aluminum alloys. Iron, copper, manganese, silicon, magnesium, and zinc are examples of elements that are alloyed.
- Aluminum and heated water react rapidly.
- The substance is reactive with sodium hydroxide.
- Aluminum is attacked slowly by most dilute acids but dissolves rapidly in concentrated hydrochloric acid. However, concentrated nitric acid can be transported in aluminum tank vehicles because it immobilizes the metal.
- Alkalies such as sodium and potassium hydroxide vigorously attack even extremely purified aluminum to produce hydrogen and the aluminate ion.
- Due to its high affinity for oxygen, finely divided aluminum will fire in carbon monoxide or carbon dioxide, producing aluminum oxide and carbide, if ignited; however, at temperatures up to red heat, aluminum is inert to sulfur.
Chemical Reaction of Aluminum
- Reaction of Aluminium with Air: Aluminum metal generally does not react with air because its surface is covered with a thin layer of oxide that protects it from air attack. However, if the oxide layer is compromised and the aluminum metal is exposed, it will react with oxygen again to produce amphoteric oxide (Aluminium (lll) Oxide), Al2O3.
4Al (s) + 3O2 (l) → 2Al2O3 (s)
- Reaction of Al with Acids: Aluminium reacts readily with mineral acids to form solutions containing aquated Al (lll) ion along with the liberation of hydrogen gas, H2. It dissolves, for instance, in hydrochloric acid (HCl), releasing dihydrogen gas.
2Al (s) + 6HCl (aq) → 2Al3+ (aq) + 6Cl– (aq) + 3H2 (g)
If exposed to Nitric Acid, it will produce a passive oxide coating of Aluminum Oxide on its surface to prevent further damage.
Al2O3 +6HNO3 → 2Al(NO3)3 + 3H2O
- Reaction of Aluminium with Alkalis: Aluminium reacts with alkalis to produce aluminates along with the release of H2 gas. Due to the similar electronegativity of oxygen and aluminum, aluminium can form covalent bonds with oxygen. This can be considered a major factor in the formation of aluminates. Aluminum, for instance, reacts with a heated, concentrated sodium hydroxide solution to produce a colorless solution of sodium tetrahydroxoaluminate and dihydrogen gas.
2Al (s) + 2NaOH (aq) + 6H2O → 2Na+ (aq) + 2[Al(OH)4]– + 3H2 (g)
Uses of Aluminum
- Aluminum is typically utilized in the form of an alloy due to its lack of durability. In contrast, copper, magnesium, manganese, and silicon alloys are lightweight yet robust. They are indispensable to the production of aircraft and a diversity of other modes of transportation.
- Numerous products, including cans, foils, kitchen utensils, window frames, beer kegs, and airplane components, contain luminium. This is due to its unique characteristics. It has a low density, is non-toxic, possesses a high thermal conductivity, possesses exceptional corrosion resistance, and can be cast, machined, and shaped with ease. Additionally, it is nonmagnetic and nonsparking. It is the second most malleable and sixth most ductile of all metals.
- Aluminum structural components are indispensable to the aerospace industry, as well as other transportation and construction sectors that require light weight, durability, and strength.
- Aluminum is used more than any other metal except iron. Pure aluminum readily combines with numerous elements, including copper, zinc, magnesium, manganese, and silicon, to produce alloys.
- On the reverse side of a sheet of float glass, a thin reflective coating of aluminum is applied to create nearly all contemporary mirrors. Additionally, telescope mirrors are coated with a thin layer of aluminum.
- Electrical transmission lines and packaging (tins, foil, etc.) are additional applications.
- In the 1960s, aluminum was widely adopted for domestic electrical wiring in the United States due to its superior conductivity and relatively low cost compared to copper. Unfortunately, the greater coefficient of thermal expansion and the tendency of the material to creep under constant, sustained pressure led to the connection becoming slack; galvanic corrosion increased the electrical resistance.
- The most recent advancement in aluminum technology is the production of aluminum foam by adding a compound (a metal hybrid) that emits hydrogen gas to molten aluminum. Before this can be accomplished, the molten aluminum must be thickened by adding aluminum oxide or silicon carbide fibers. The outcome is a substantial foam used in traffic tunnels and space shuttles.
- Aluminum is an excellent conductor of electricity and is frequently used in transmission lines. It is less expensive than copper and nearly twice as effective as copper per unit of weight.
- Aluminum forms a highly reflective coating for both light and heat when evaporated in a vacuum. It does not degrade, as would a silver coating. These aluminium compounds have numerous applications, such as telescope mirrors, decorative paper, packaging, and toys.
- When evaporated in a vacuum, aluminum creates a highly reflective covering for both light and heat. It does not corrode in the same manner that silver does. Aluminum compounds are useful for packaging, telescope mirrors, decorative paper, and toys.
Health Effects of Aluminum
Aluminum is both one of the most commonly used metals and one of the most commonly found elements in the earth’s crust. Due to these facts, aluminum is commonly regarded as a harmless substance. However, exposure to excessive concentrations can still cause health problems.
- Ions are the water-soluble form of aluminum that is responsible for the detrimental effects. Typically, they are found in aluminum solutions in combination with other ions, such as aluminum chloride.
- Aluminum can be absorbed through the digestive tract, the lungs, and the skin. Long-term exposure to significant concentrations of aluminum can have severe health consequences, including: Central nervous system damage – Dementia – Memory loss – Lethargy – Severe quivering
- The presence of aluminum in water in certain work environments, such as mines, is hazardous. Aluminum particles inhaled by workers in factories where aluminum is used in the production process can cause respiratory problems.
- When aluminum penetrates the body during kidney dialysis, it can cause issues for patients with kidney disease.
- Pulmonary fibrosis and lung injury have been associated with the inhalation of finely divided aluminum and aluminum oxide powder. This effect, known as Shaver’s Disease, is exacerbated by the presence of silica and iron oxides in the inhaled air.
Environmental Effects of Aluminum
- Aluminum may accumulate in vegetation and pose health risks to animals consuming these plants.
- It appears that acidified lakes contain the greatest levels of aluminum. Due to the reactions of aluminum ions with proteins in the gills of fish and the embryos of frogs, the number of fish and amphibians in these lakes is declining.
- High concentrations of aluminum affect not only fish, but also birds and other animals that consume contaminated fish and invertebrates, as well as animals that breathe in aluminum through the air. Eggshell thinning and underweight offspring are the results of birds consuming contaminated fish. There may be respiratory problems, weight loss, and a decrease in activity in animals that inhale aluminum through the air.
- Another negative environmental effect of aluminum is that its ions can react with phosphates, making phosphates less accessible to water organisms.
- In addition to acidified lakes and air, high concentrations of aluminum can also be found in acidified soils’ groundwater. There are significant indications that the presence of aluminum in groundwater can harm the roots of trees.
- Each stage of the aluminium manufacturing process presents its own environmental challenges. The greatest obstacle is the emission of greenhouse gases. These gases result from the smelters’ electrical consumption and byproducts of processing. The most dangerous of these gases are perfluorocarbons, which are produced during the smelting process. Sulfur dioxide emissions are one of the primary causes of acid rain.
- Biodegradation of metallic aluminium is extremely uncommon; the majority of aluminium-corroding organisms generate corrosive wastes rather than directly attacking or consuming aluminium. Compact discs’ aluminum can be consumed by the fungus Geotrichum candidum. The fungus Cladosporium resinae and the bacterium Pseudomonas aeruginosa are commonly found in aircraft fuel containers that use kerosene-based fuels (not avgas), and laboratory cultures can degrade aluminum.
Learn the basics about aluminium – anodising it and the uses of aluminium, when learning about metals and their reactivity as a part of environmental chemistry. Watch out this interesting video.
References
- Enghag, Per (2008). Encyclopedia of the Elements: Technical Data – History – Processing – Applications. John Wiley & Sons. pp. 139, 819, 949. ISBN 978-3-527-61234-5
- https://byjus.com/chemistry/chemical-properties-of-aluminium/
- https://www.sciencehistory.org/distillations/aluminum-common-metal-uncommon-past
- Rosseland, B.O.; Eldhuset, T.D.; Staurnes, M. (1990). “Environmental effects of aluminium”. Environmental Geochemistry and Health.
- https://en.wikipedia.org/wiki/Aluminium#Natural_occurrence
- https://www.sciencehistory.org/distillations/aluminum-common-metal-uncommon-past
- https://www.lenntech.com/periodic/elements/al.htm
- https://www.rsc.org/periodic-table/element/13/aluminium#:~:text=Aluminium%20is%20a%20silvery%2Dwhite,because%20of%20its%20particular%20properties.
- https://pubchem.ncbi.nlm.nih.gov/element/Aluminum#section=Sources

