Allotropes occur when one element may exist in more than one physical configuration, providing distinct allotropes diverse structures and characteristics and, hence, different usage in daily life. Each carbon atom may form a maximum of four bonds. As a result, it might take several shapes. Carbon has three major allotropes: graphite, diamond, and buckminsterfullerene (buckyballs). Allotropes of carbon only consist of carbon atoms. Thus, all of them are the purest form of carbon.
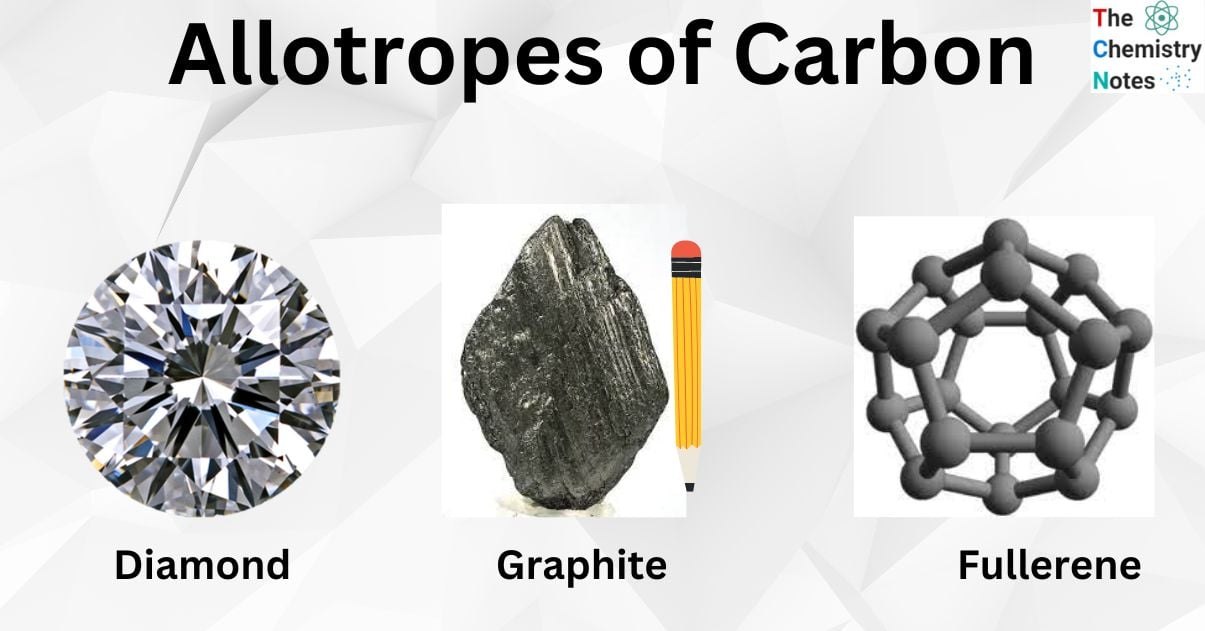
Important Allotropes of Carbon
Carbon in allotropic amorphous forms: Carbon in amorphous allotropic forms includes: Coal, Charcoal made from Coke Wood. Charcoal from animals, Charcoal made from sugar, Lampblack, and Gas Carbon
Diamond
It is a kind of carbon in which the atoms are arranged in a diamond cubic crystal form. Graphite, another solid form of carbon, is the chemically stable form of carbon at normal temperature and pressure, although diamond almost never converts to it. Diamond is the hardest and most thermally conductive natural mineral, making it excellent for cutting and polishing equipment in the industry.
Graphite
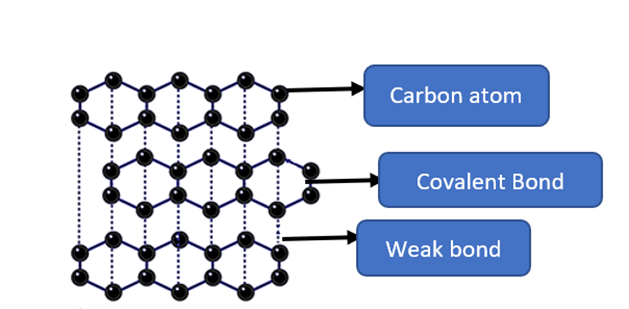
Each carbon atom in graphite is covalently bonded to three other carbon atoms in the same plane due to sp2 hybridization. As a result, planar hexagonal rings are formed. The Carbon-Carbon bond in this ring is 142 pm long. Hexagonal rings create the layers. The layers are held together by Van der Waals force, whereas 142 pm separates them. Graphite is soft and lubricious because these layers may glide over one another.
Fullerene
As opposed to graphite or diamond, which have remarkable edges or surface securities that attract others, fullerenes are the purest form of carbon. Fullerene is produced by heating graphite in an electric bend in an inactive gas, for example, helium or argon, when a filthy substance is formed by the accumulation of tiny atoms.
Structure and Uses of Allotropes of Carbon
Graphite
In graphite, carbon atoms are organized in planar layers. Inside the layers, carbon atoms are organized in hexagons. Each carbon atom is linked to three other carbon atoms by strong covalent bonds. A p orbital is occupied by the fourth electron of each carbon atom. In each planar layer, the p orbitals of each carbon atom overlap sideways. A cloud of delocalized electrons arises above and below the plane of the carbon rings. As these electron clouds interact, they generate extended delocalized electron rings. Van der Waals forces hold the carbon atom layers together.
Uses
- It is utilized in the production of carbon arcs and electrodes.
- It is a lubricant for high-temperature machinery.
- This substance is used to make lead pencils. Graphite powder and clay are mixed together and shaped into sticks. These sticks are used to create pencils.
- It serves as a moderator in atomic reactors.
- It’s used as a reducing agent in steel manufacture.
- It is used to make high-strength composites.
- It is used in the production of Crucibles, which can withstand extremely high temperatures. Also, since it is inert to acids and alkalis, it is used to build crucibles.
- In the steel industry, graphite is utilized as a reducing agent.
Diamond
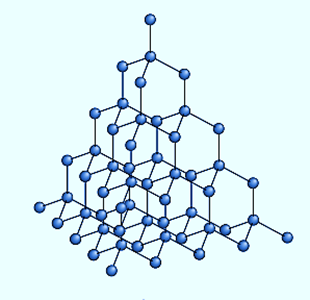
Diamond’s crystal structure is an endless three-dimensional array of carbon atoms, each of which creates a structure with equal angles between the bonds. If the bond ends are joined, the structure is a tetrahedron, a three-sided pyramid with four faces (including the base). Every carbon atom is covalently connected to four other carbon atoms at the four corners of the tetrahedron.
The single-bond length is the distance between carbon atoms along the bond, which is 1.54 × 10−8 cm. The diamond’s space lattice can be visualized as puckered hexagonal (six-sided) rings that lie roughly in one plane, the crystal’s natural cleavage plane; and these sheets of hexagonal, puckered rings are stacked in such a way that the atoms in every fourth layer lie in the same position as those in the first layer.
Uses
- Diamonds are used in jewelry because of their extraordinary radiance.
- Because diamond is the hardest substance on the planet, it may be used in a variety of applications such as industrial cutting tools, milling tools, grinding tools, saws, and so on.
- Because of its scarcity, minimal chemical reactivity (thus, high biological compatibility), and, of course, beauty, it is a desirable material in the jewelry business.
- Eye surgeons utilize sharp-edged diamonds to remove cataracts from the eyes with exceptional precision.
- Diamond dies are used to create ultra-thin metal wires like tungsten.
Fullerene
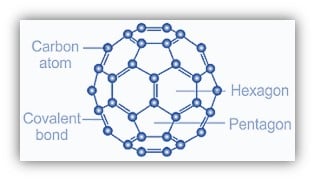
The fullerene, C60, an allotropes of Carbon is made up of fused five and six-membered carbon rings. Each six-membered ring is encircled by hexagons and pentagons of carbon, which are fused to five hexagons. As a result of this structural theme, each hexagon is like the base of a bowl; the three pentagons fused to this ring, joined by hexagons, compel the structure to curve, resulting in a dome-like structure that finally curls around itself to form a spherical. Fullerene, C60 has the form of a soccer ball. All 60 carbon atoms are the same.
Nanotubes, a kind of fullerene, are made up of molecular-scale carbon tubes that are stacked in layers similar to graphite.
Carbon nanotubes have an unusually high melting point due to the strong covalent bonds that bind each carbon atom to three others. Moreover, each carbon atom now possesses an additional electron, creating a sea of delocalized electrons inside the tube, allowing nanotubes to conduct electricity.
Read also: Biological Role of Carbon
Uses
- Fullerene is used as a synthetic lubricant.
- It absorbs gases.
- It is used in the biomedical sector for a variety of purposes, including medication delivery systems and light-activated antibacterial treatments.
- Fullerene is an excellent electrical conductor and is utilized in batteries and electronic equipment.
- Fullerenes are used in a variety of cosmetic-related goods.
- Carbon nanotubes are made from graphene sheets.
- Fullerene is a chemical that is utilized in nanotechnology.
- Fullerene-based conductors are used.
Other Allotropes of Carbon
Some of few allotropes of carbon other than graphite, diamond, and fullerene includes:
- Linear acetylenic carbon (Carbyne)
- Amorphous carbon
- Lonsdaleite: Also known as hexagonal diamond.
- Graphene is the fundamental structural component of allotropes, nanotubes, charcoal, and fullerenes.
- Q-carbon: These carbon allotropes have ferromagnetic crystal structures that are tougher and brighter than diamonds.
Learn the basics about bucky balls, graphene and nano tubes
References
- McNaught, A.D.; Wilkinson, A., eds. (1997). “Diamond-like carbon films”. IUPAC Compendium of Chemical Terminology (2nd ed.). Oxford, UK: Blackwell Scientific Publications.
- https://collegedunia.com/exams/allotropes-of-carbon-chemistry-articleid-4008
- https://www.bbc.co. uk/bitesize/guides/ zjfkw6f/revision/4
- https://www.geeksforgeeks.org/allotropes-of-carbon/
- https://edu.rsc.org/infographics/allotropes-of-carbon/4012885.article
- https://www.embibe.com/exams/allotropes-of-carbon/
- https://www.clearias.com/carbon-and-its-allotropes/


I really love the content thank you