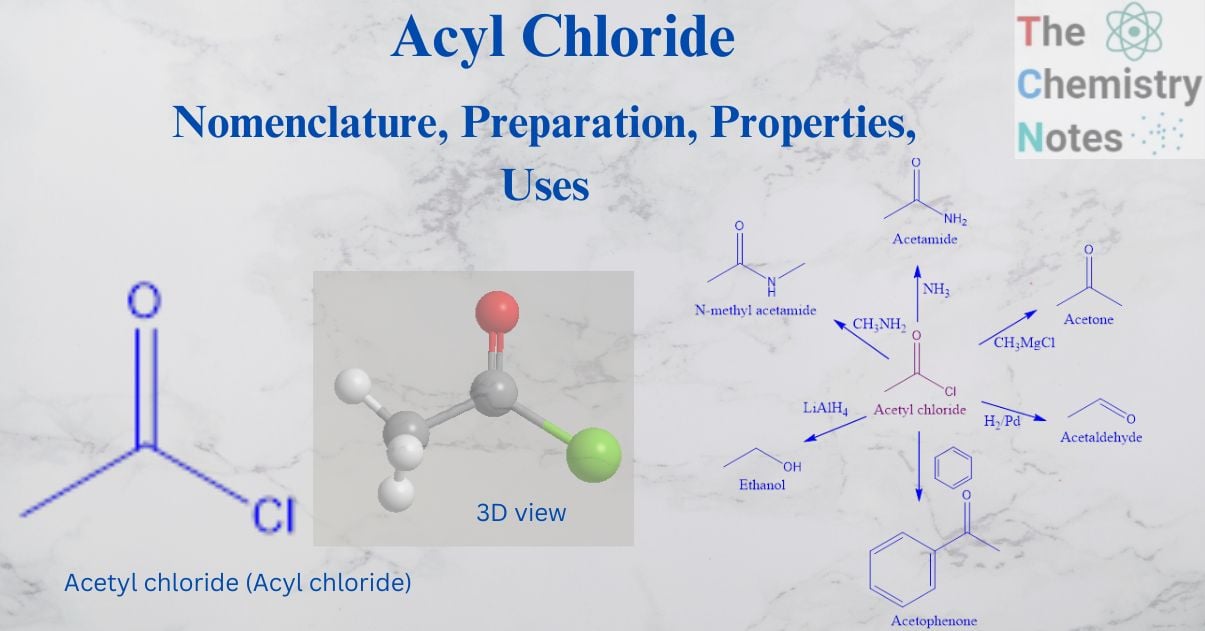
Acyl chloride is an important derivative of carboxylic acid. It has an sp 2 hybridized carbonyl group with an O atom connected to a C atom through a double bond in a planar form with bond angles of about 120o.
It is an organic molecule composed of a chlorine atom and an acyl group. The molecular formula for acyl chloride is RCOCl, where R is an alkyl group. The molecular formula of RCO defines an acyl group as a functional group.
Nomenclature
IUPAC names of acyl chlorides are derived from those of the parent acid by replacing the ending -ic in the name of the acid with -yl and adding the word chloride.
| Formula | Common name | IUPAC name |
| HOCl | Formyl chloride | Methanoyl chloride |
| CH3COCl | Accetyl chloride | Ethanoyl chloride |
| C2H5COCl | Propionyl chloride | Propanoyl chloride |
| C3H7COCl | Butyryl chloride | Butanoyl chloride |
Acetyl chloride is the most important member of this class.
Preparation of acyl chloride
Acid chlorides, commonly known as acyl chlorides, have the general formula R-COCl. They are formed by replacing a -OH group with a – Cl atom.
1. From acid
Heating carboxylic acids with phosphorus trichloride (PCl3), phosphorus penta chloride (PCl5), or thionyl chloride (SOCl2) yields acyl chlorides.
3 CH3COOH + PCl3 → 3 CH3COCl + H3PO3
Acetic acid Phosphorus trichloride Acyl chloride
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl\
Phosphorus pentachloride
CH3COOH + SOCl2 → CH3COCl + HCl + SO2
Thionyl chloride
2. From salt
a. Acyl chlorides can be prepared by reacting sodium salts of carboxylic acids with phosphorous trichloride (PCl3) or thionyl chloride (SOCl2).
3 CH3COONa + PCl3 → 3 CH3COCl + Na3PO3
b. Acyl chloride is produced industrially by distilling calcium acetate ( (CH3COO)2Ca) and sodium acetate ((CH3COO)2Na ) with sulphuryl chloride ( SO2Cl2 ).
(CH3COO)2Na + SO2Cl2 → 2 CH3COCl + Na2SO4
(CH3COO)2Ca + SO2Cl2 → 2 CH3COCl + CaSO4
Laboratory preparation
Acyl chloride is synthesized in the laboratory by reacting phosphorus tri chloride or penta chloride with glacial acetic acid. The following equipment is used to make acyl chloride:
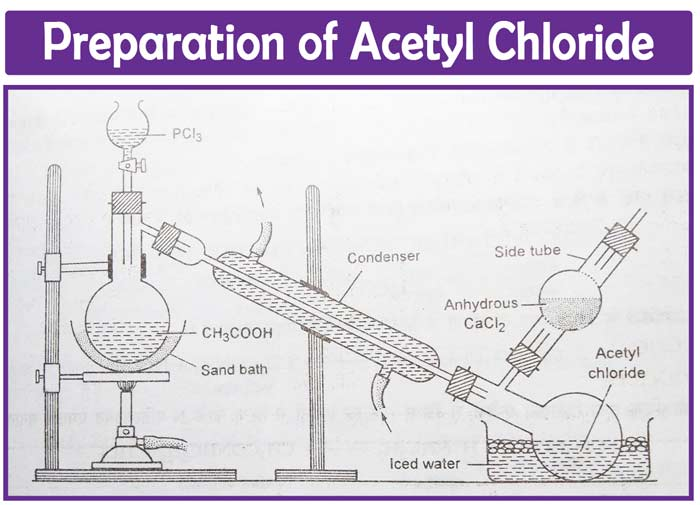
Preparation of acyl chloride
Image source: https://chemistrypage.in/acetyl-chloride-and-acetyl-chloride-structure/
Phosphorous trichloride is progressively added to glacial acetic acid in a distillation flask using a dropping funnel. In the early stages, it may be essential to cool the flask. The temperature gradually rises to 40- 50 degrees Celsius. The temperature is raised to boiling as the reaction slows. The distilled acetyl chloride is collected in an ice-cold receiver.
Physical properties
I. The lower members are colorless, volatile liquids with an offensive smell. Higher members are colorless liquids.
II. They dissolve in organic solvents such as alcohol, ether, and chloroform.
III. Because of their inability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than their corresponding acids.
IV. The relative density of acyl chloride is 1.104.
V. It decomposes quickly in water thus, it absorbs water vapor from the air and decomposes to produce hydrogen chloride.
The polarity of acyl chloride
The chemical reactions of organic compounds containing an acyl group are determined by the type of bond (polar or nonpolar) formed between the acyl group’s carbonyl carbon and the substituent attached to it. Acyl compounds are organic compounds that contain an acyl group. Acyl compounds are categorized into two groups based on their polarity: nonpolar carbonyl compounds and polar acyl compounds. In acyl chlorides, the carbonyl carbon is directly attached to the chlorine. As chlorine is more electronegative than carbon, such bonds are polar.
Nucleophilicity and reactivity
The magnitude of the + charge on the carbonyl carbon is determined by the substituent’s electron-releasing or electron-attracting power. Acid chlorides and anhydrides’ substituent groups can withdraw electrons from the carbonyl carbon, making these compounds more reactive than carboxylic acids. In acyl chloride, the electron-withdrawing inductive effect is not stabilized by electron pair donation by other groups. So the electron-withdrawing inductive effect of chlorine makes it more electrophilic and reactive towards nucleophilic acyl substitution.
Chemical properties
Acyl chlorides are the most reactive carboxylic acid derivatives. Acid halides can be converted into other acyl compounds via nucleophilic acyl substitution due to their high reactivity. They can easily undergo nucleophilic acyl substitutions to produce acid anhydrides, esters, and amides. Methanoyl chloride (CHOCl) and ethanoyl chloride (CH3COCl) are common acyl chlorides. The chemical characteristics of acyl chlorides are as follows:
1. Basic character
Since the chlorine atom is ineffective at stabilizing a positive charge, acyl chlorides are significantly less basic.
2. Chlorination
Chlorine reacts with acetyl chloride, replacing one or more hydrogen atoms in the methyl group.
CH3COCl + Cl2 → ClCH2COCl ( Monochlorinated acetyl chloride) + HCl
3. Reaction with alcohol
Acyl chloride reacts with alcohol to give ester.
CH3COOCl + HOC2H5 → CH3COOC2H5 ( Ethyl acetate) + HCl
4. Reaction with ammonia
A strong solution of ammonia in water reacts with acyl chloride to produce amide.
CH3COCl + HNH2 → CH3CONH2 ( acetamide) + HCl
5. Reduction (Rosenmund reduction)
It reacts with hydrogen in the presence of finely divided palladium to form acetaldehyde.
CH3COCl + H2 → CH3COH (acetaldehyde) + HCl
6. Reaction with lithium aluminum hydride
Lithium aluminum hydride (LiAlH4) reduces it to produce ethanol.
CH3COCl → CH3CH2OH ( ethanol) + HCl
7. Fredal craft reaction
Acyl chloride reacts with benzene in the presence of anhydrous aluminum chloride to form acetophenone. Due to the tendency of complexation with carbonyl groups and water to hydrolyze the Al salts, this reaction necessitates an excess of AlCl3.
C6H6 + CH3COCl → C6H5COCH3 (acetophenone) + HCl
8. Reaction with Ether
In the presence of anhydrous zinc chloride, it reacts with di ethyl ether to give ethyl chloride and ethyl acetate.
CH3COCl + C2H5OC2H5 (diethyl ether)→ C2H5Cl ( ethyl chloride)+ CH3COOC2H5 (ethyl acetate)
9. Reaction with grignard reagent
It reacts with the Grignard reagent to form Ketone.
CH3COCl + CH3MgCl → CH3COCH3 (acetone) + MgCl2
10. Reaction with amines
Acid amides are formed when acid chlorides react with primary and secondary amines.
CH3COCl + CH3NH2→ CH3CONHCH3 (N-methyl acetamide) + HCl
Uses of Acyl chloride
- In chemistry, acetyl chloride is employed as a reagent.
- It is used in the manufacture of agrochemicals.
- It is utilized in the synthesis of organic compounds.
- It is used in the production of pharmaceuticals.
- Acetic anhydride, acetamide, acetaldehyde, and other carbonic chemicals are produced by using acetyl chloride.
- As an acetylating reagent in the production of dyes and pharmaceuticals.
Impact of acyl chloride on health
1. It can result in a burning sensation, blisters, and severe skin burns.
2. Inhalation causes coughing, sore throat, burning sensation, and shortness of breath.
3. They are called lachrymatory compounds because they can react with water on the eye’s surface, producing hydrochloric and organic acids that irritate the eyes.
4. In a fire, it emits irritating or hazardous gases. Many reactions can result in a fire or explosion.
References
- Smith M. & March J. (2001). March’s advanced organic chemistry : reactions mechanisms and structure (5th ed.). Wiley.
- R.D Madan, B.S. Bist 2005. ISC Chemistry book -II, Fourth edition. S. Chand and Company Ltd.
- https://byjus.com/chemistry/acid-chloride/.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Acid_Halides/Synthesis_of_Acid_Halides/Preparation_of_Acyl_Chlorides.
- https://chemistrypage.in/acetyl-chloride-and-acetyl-chloride-structure/.
- https://www.slideshare.net/mizakamaruzzaman/chapter-5-acyl-chloride.
