Organic reactions can be better understood by summarizing the overall reaction into a series of steps known as a reaction mechanism. Organic reactions are governed by factors that are essentially the same as those that govern any chemical reaction. The stability of reactants and products is influenced by factors unique to organic reactions, including aromaticity, hyperconjugation, and conjugation, as well as the presence and stability of reactive intermediates like free radicals, carbocations, and carbanions.

Organic reactions, like all chemical reactions, involve forming and breaking chemical bonds. The two ways that covalent bonds can rupture are as follows:
- Homolytic fission
- Heterolytic fission
Homolytic fission
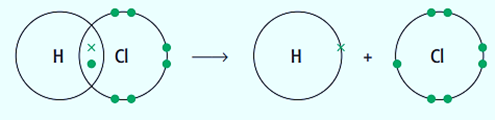
When a covalent bond breaks, both atoms at each end of the bond lose one of the two electrons that were originally present. Free radicals are the species that are created when a bond breaks homolytically. Using the following equation, we can demonstrate the production of free radicals:
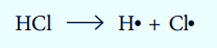
Cl and H are both free radicals. Free radicals are extremely reactive and all have an unpaired electron (represented by the dot).
- Free radicals are created as part of the initiation process of this kind of reaction. To create two free radicals, a covalent bond must be broken, which requires energy input.
- The resulting radicals can then attack the molecules of the reactant, producing additional free radicals. These are known as propagation steps. They resemble a chain reaction that only comes to an end when free radicals interact with one another.
- When two free radicals interact, a molecule is created without the production of any additional free radicals. Consequently, this is known as a termination step.
Heterolytic fission
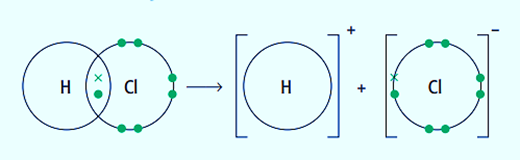
The “uneven” breaking of a covalent bond is heterolytic fission. The more electronegative atom in a covalent bond takes both electrons in heterolytic fission. An equation can be used to depict this type of bond breaking.
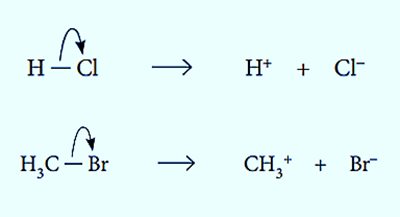
C-X bonds, where X is an atom with a higher electronegativity value than carbon, are capable of being broken apart through heterolytic fission.
In organic reactions, a few patterns recur frequently, many of which reflect the interactions between nucleophiles and electrophiles.
Electrophiles
Electrophiles are positively charged or neutral species that are electron-deficient and can accept a pair of electrons. These are referred to as “electron-loving species.”
The words “electro,” which comes from the word “electron,” and “phile,” which means “loving,” make up the term electrophile.
They love to accept electrons because they lack electrons. They are neutral or positively charged. They draw electrons to them. The density affects how quickly electrons move. From a high-density area to a low-density area, they move.
Both electrophilic addition and electrophilic substitution reactions take place on them. A Lewis acid is another name for an electrophile.
For Example: Methyl carbocation, ethyl carbocation.
Nucleophile
A nucleophile is a reagent that has a negative charge or a single pair of electrons. A nucleophile seeks out areas where there are electron shortages because it is electron-rich. In the Lewis model, nucleophiles serve as species that can donate two electrons, or Lewis bases.
The term nucleophile can be split into “nucleo” derived from the nucleus and “phile” which means loving. They love nuclei because they are electron-rich. They are neutral or negatively charged. They donate electrons. The density affects how quickly electrons move. From a low-density to a high-density area, they move.
Both nucleophilic addition and nucleophilic substitution reactions take place on them.
A Lewis base is another name for a nucleophile.
Ammonia, for instance, is a stronger nucleophile than water because nitrogen is less electronegative than oxygen. Compared to the lone pair of electrons on oxygen in water, the lone pair of electrons on nitrogen in ammonia can be given more readily.
For Example: Methyl carbanion, chloride ion.
Types of organic reaction
Chemical processes involving organic compounds are known as organic reactions. The fundamental types of organic chemistry reactions include redox reactions, photochemical reactions, pericyclic reactions, elimination reactions, and substitution reactions. In the process of creating new organic molecules, organic reactions are used. Many man-made chemicals, including medicines, plastics, food additives, and textiles, are produced through organic reactions.
Combustion of organic fuels and the saponification of fats to produce soap are the two oldest organic reactions. With the Wöhler synthesis in 1828, modern organic chemistry got its start. The Nobel Prize in Chemistry has been awarded for the discovery of specific organic reactions, such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979, and olefin metathesis in 2005.
Addition Reaction
During addition reactions, two reactant molecules combine to form a single product.
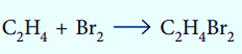
Elimination Reaction
Elimination reactions involve the removal of a small molecule from a larger one.
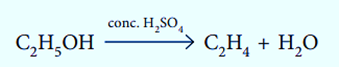
Substitution Reaction
The replacement of one atom, or a group of atoms, by another occurs in substitution reactions.
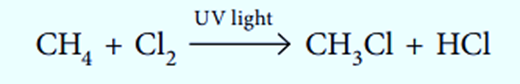
Hydrolysis Reaction
A molecule is broken down by water during hydrolysis. Acid or alkali frequently speed up this kind of reaction.
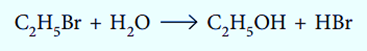
Oxidation-Reduction Reaction
The loss of electrons from a species is referred to as oxidation.
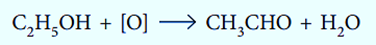
Changes in the number of oxygen atoms at a specific position in the hydrocarbon skeleton or in the number of bonds between carbon and oxygen at that position are frequently used to identify oxidation-reduction reactions, which are frequent in organic chemistry. An oxidation occurs when either value increases, whereas a reduction occurs when either value decreases. On the other hand, an increase in the proportion of hydrogen atoms in a hydrocarbon is frequently a sign of a decrease.
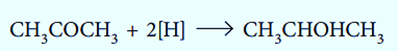
References
- Strategic Applications of Named Reactions in Organic Synthesis Laszlo Kurti, Barbara Czako Academic Press (March 4, 2005) ISBN 0-12-429785-4
- J. Clayden, N. Greeves & S. Warren “Organic Chemistry” (Oxford University Press, 2012)
- March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2
- Robert T. Morrison, Robert N. Boyd, and Robert K. Boyd, Organic Chemistry, 6th edition, Benjamin Cummings, 1992
- https://www.savemyexams.co.uk/as/chemistry/cie/22/revision-notes/3-organic-chemistry/3-1-an-introduction-to-as-level-organic-chemistry/3-1-5-characteristic-organic-reactions/
- https://byjus.com/chemistry/types-of-organic-reactions/
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/23%3A_Organic_Compounds/23.05%3A_Common_Classes_of_Organic_Reactions
- https://www.studysmarter.us/explanations/chemistry/organic-chemistry/
- https://byjus.com/chemistry/difference-between-electrophile-and-nucleophile/
