When a chemical change occurs, a chemical reaction is said to have taken place. In other words, a chemical reaction causes a chemical change in the reactants. Thus, we can say that both chemical change and chemical reaction imply the same thing.
Types of Chemical Reactions
There are many types of chemical reactions. All the available chemical reactions are classified into different types according to their nature. They are discussed below:
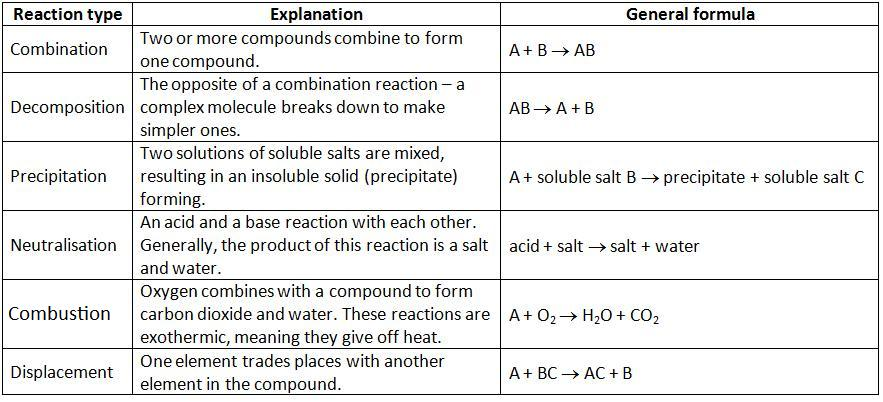
Decomposition or Analysis reaction
A decomposition reaction is the process of breaking up a substance into two or more simpler constituents with the help of heat, light, electricity, etc. The general form of the decomposition reaction, otherwise known as the analysis reaction, is:
XY → X + Y
Most of the decomposition reactions are endothermic.
Examples of Decomposition Reactions
- When heated, mercuric oxide decomposes into mercury and oxygen.
2HgO (s) → 2Hg (l) + O2 (g) [ When heat is applied]
On heating, other metal oxides also undergo the decomposition process. Depending on the type, metal oxides experience decomposition at different temperatures.
- When one or more of the products remains a compound even after a reaction, it is also known as a decomposition reaction. For example, calcium carbonate (CaCO3) generates calcium oxide (CaO) and carbon dioxide (CO2) when decomposed.
CaCO3 (s) → CaO(s) + CO2 (g)
- The electrolytic decomposition of water is also an example of a decomposition reaction. In this reaction, water (H2O) breaks down into hydrogen (H2) and oxygen (O2). Both of these are gases, which have different properties than water.
H2O (l) → H2 (g) + O2 (g)
Synthesis or Combination reaction
The process of two or more substances reacting to form a new compound is known as a synthesis reaction. The general form of the synthesis reaction, otherwise known as the combination reaction, is:
X + Y → XY
Most of the synthesis reactions are exothermic.
Examples of Synthesis Reactions
- When Solid sodium metal reacts with chlorine gas to produce solid sodium chloride.
2 Na (s) + Cl2 (g) → 2 NaCl (s)
- When metals (I and II group metals) are combined with oxygen to generate their corresponding oxides, this is another example of a synthesis reaction.
2 Mg (s) + O2 (g) → 2 MgO (s) [when burned]
- When iron and sulfur react, they will form iron (II) sulfide; this is also an example of a synthesis reaction.
8 Fe + S8 → 8 FeS
Single Displacement or Replacement Reaction
Single displacement reactions are chemical reactions in which a more reactive element displaces a less reactive element from its aqueous salt solution. In these reactions, products can be determined through reactivity series. It is a series where elements are arranged in decreasing order of their reactivity. The general form of the single displacement reaction, otherwise known as the single replacement or substitution reaction, is:
X + YZ → XZ + Y
Here X replaces Y; only metals can replace metal, and nonmetals will replace nonmetals.
So if X and Y are nonmetals, X will replace Y and form a new product XZ.
Similarly, if X and Y are metals, X will replace Y and form a new product called XZ.
Displacement does not not occur when:
X is metal and Y is nonmetal and vice versa.
Examples of Single Displacement Reactions
- The reaction of potassium with magnesium chloride is an example of a single displacement reaction. In this reaction, potassium displaces magnesium from its salt because potassium is more reactive than magnesium. Potassium is present at the top of the reactivity series and is the most reactive element.
2 K + MgCl2 → 2 KCl + Mg
- The reaction of zinc with tin chloride is another example of a single displacement reaction. In this reaction, zinc, being a more reactive metal, replaces tin, leading to the formation of zinc chloride.
Zn + SnCl2 → ZnCl2 + Sn
Double Displacement or Metathesis Reaction
In double displacement reactions, two aqueous ionic compounds exchange ions (mostly cations) to form completely different compounds. The exchange during this type of reaction is either of cations or anions. It’s never both at the same time.
The general form of the double displacement reaction, otherwise known as the metathesis, is:
XY + ZA → XZ + YA
In this reaction, X and Z are positively charged cations, while Y and A are negatively charged anions. Double-displacement reactions generally occur between substances in aqueous solutions.
Examples of Double Displacement Reactions
- Potassium nitrate (KNO3) reacts with aluminum chloride (AlCl3) and forms aluminum nitrate (Al(NO3)3) and potassium chloride (KCl).
KNO3 (aq) + AlCl3 (aq) ↔️ Al(NO3)3 (aq) + KCl (s)
- Sodium chloride (NaCl) and silver nitrate (AgNO3) react to form sodium nitrate (NaNO3) and silver chloride (AgCl).
NaCl (aq) + AgNO3 (aq) ↔️ NaNO3 (aq) + AgCl (s)
Combustion Reaction
The most basic kinds of chemical reactions frequently include the combustion process. Compounds are burned and released in the form of heat and light energy. Oxygen is one of the necessary reactants in combustion reactions.
Examples of Combustion Reaction
When methane is burned, it gives off carbon dioxide and water.
CH4 + 2 O2 → CO2 + 2 H2O
Another example of a combustion reaction is the burning of naphthalene.
C10H8 (g) + 12 O2 (g) → 10 CO2 (g) + 4 H2O (g)
Acid-base Reaction or Neutralization Reaction
An acid-base reaction is a type of double displacement reaction that occurs between an acid and a base. It is the process by which salt and water form when a reaction occurs between acid and base.
The general form of the acid-base reaction, otherwise known as the neutralization reaction, is:
HX + YOH → H2O + YX
The H+ ion in the acid reacts with the OH– ion in the base to form water and an ionic salt:
Examples of Acid-Base Reactions
The reaction between potassium hydroxide (KOH) and nitric acid (HNO3).
KOH + HNO3 → KNO3 + H2O
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH).
HCl + NaOH → NaCl + H2O
The reaction between hydrobromic acid (HBr) and sodium hydroxide (NaOH).
HBr + NaOH → NaBr + H2O
Hydrolysis Reaction
Hydrolysis is the term for any decomposition process that occurs with water action. Hydro means water, and lysis means to break down. In general, hydrolysis is the interaction of positive and negative ions of salt with water, resulting in acidity or basicity in the solution.
The general form of the hydrolysis reaction is:
XY + H2O → XH + YOH
Examples of Hydrolysis Reactions
The reaction between sodium carbonate (Na2CO3) and heavy water (2H2O).
Na2CO3 + 2 H2O → 2 NaOH + H2CO3
Precipitation Reaction
It is the process of forming one of the products as an insoluble product by mixing two compounds from their aqueous solution. Precipitation reactions in aqueous solutions are mostly double-decomposition reactions. Like the neutralization reaction, it also qualifies as a double displacement.
Examples of Precipitation Reactions
The reaction between hydrochloric acid (HCl) and silver nitrate (AgNO3).
NaCl (aq) + AgNO3 (aq) → AgCl (s)↓ + NaNO3 (aq)
In the above reaction, silver (I) chloride (AgCl) precipitates out and settle at the bottom.
Salt-Acid Displacement Reaction
In this reaction, the salt of a volatile acid interacts with nonvolatile acids such as sulfuric acid to give the volatile acid. Like the precipitation reaction, it is also a double decomposition reaction.
The general form of salt-acid displacement reaction is:
XY + ZA → XZ + YA
or, XY + ZA → XA + YZ
Examples of Salt-Acid Displacement Reactions
2 NaCl + H2SO4 (conc) → Na2SO4 + 2 HCl
FeS + H2SO4 (conc) → FeSO4 +H2S
Redox Reaction
Redox reactions are oxidation-reduction processes in which molecules, atoms, or ions alter their oxidation numbers by gaining or losing electrons. An atom that loses an electron is said to be oxidized, and one that gains an electron is said to have been reduced. Redox processes might be single-replacement, combustion, breakdown, or synthesis processes. But not all combustion processes are redox processes.
Examples of Redox Reactions
Iron (III) oxide, which has an oxidation number of +3, is created when iron (Zero) combines with oxygen (O2).
4 Fe + 3 O2 → 2 Fe2O3
The reaction between the thiosulfate ion and iodine, where I2 is reduced to I- and S2O2- is oxidized to S4O62-.
2 S2O32− (aq) + I2 (aq) → S4O62− (aq) + 2 I− (aq)
Polymerization or Chain reaction
Polymerization is a process that forms a chain-like network called a polymer when a chemical combination of relatively small units called monomers occurs. It is also known as the chain reaction.
Examples of Polymerization Reactions
When ethylene (C2H4) is polymerized, it produces polyethylene.
C2H4 + C2H4 + C2H4 → (C2H4)n
Types of Chemical Reactions Video
References
- Atkins, Peter W.; Julio de Paula (2006). Physical Chemistry (4th ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31546-8.
- https://byjus.com/chemistry/types-of-chemical-reactions/
- https://chemistrytalk.org/types-of-chemical-reactions/
- https://www.thoughtco.com/types-of-chemical-reactions/
- https://www.studysmarter.co.uk/explanations/chemistry/chemical-reactions/types-of-chemical-reactions/
- Wiberg, Egon, Wiberg, Nils and Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. ISBN 978-0-12-352651-9.
- https://letstalkscience.ca/educational-resources/backgrounders/types-chemical-reactions
- https://www.excellup.com/classten/scienceten/10_sc_ChemicalReaction2.aspx

