Nanoscience and nanotechnology rely heavily on nanomaterials. The study of nanostructures and the use of related technologies is a rapidly expanding field of study that spans several academic disciplines. It might drastically alter the production of materials and goods as well as the kinds and levels of functionality that are available.
In order to understand the novel properties of nanostructured materials, we need to understand the structure and its interrelationship with properties. The following are some important microstructural characteristics of nanomaterials:
- Grain size, distribution and morphology
- nature of grain boundaries and interphase interfaces
- Nature of intragrain defects
- Composition profiles across grains and interfaces
- Residual impurities from processing
Mechanical, thermal, electrical, melting, magnetic, catalytic, diffusive, and optical properties are just a few of the many unique characteristics of nanomaterials. These characteristics are unique to nanomaterials, which do not exist in their bulk form. The unique characteristics of nanomaterials can be related back to their relatively small size.
Some of the unique nanomaterial properties are:
Elastic properties
The bonding strength between atoms or molecules and a material’s elastic modulus are inversely correlated. The melting temperature and elastic modulus will increase in direct proportion to the bond strength. At the equilibrium separation distance, the second differential of the interatomic force-distance curve is known to be proportional to the elastic modulus. The elastic characteristics of crystalline materials are typically thought to be independent of structure (microstructure).
The elastic modulus falls as temperature rises and the mean distance between atoms increases. It is possible to treat a significant increase in vacancy and other defect concentrations as being similar to a rise in the considered temperature. It follows that a rise in defect concentration should lead to a fall in elastic modulus. However, the impact of defects on elastic modulus does not become apparent until vacancies are present in much larger concentrations. Nanomaterials may have significantly less elastic properties than bulk materials due to their extremely high defect concentration.
Melting Point
The enthalpy of fusion and melting temperature can be decreased due to the decrease in bonding energy caused by increased surface and grain boundary area in nanocrystalline materials. Atoms in a solid are known to vibrate about their mean location. With the rising temperature, the amplitude of vibration increases. Melting starts at the surface and spreads through the solid when the vibration amplitude surpasses a specific threshold of the bond length. Atoms outside of the crystal lattice are less restricted from vibrating than those at the surface and grain boundaries. The proportion of atoms found at surfaces and grain boundaries rises noticeably as grain size decreases. As a result, freestanding nanoparticles may exhibit a lower melting point than bulk materials.
Diffusivity
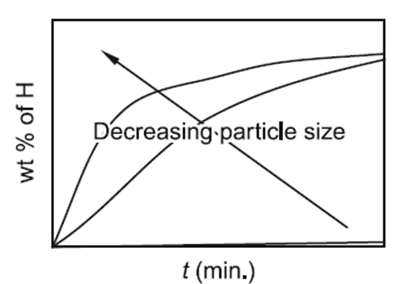
With an increase in the material’s defect content, such as vacancy concentration, the rate of diffusion increases. Because the structure is more open and faulty than the bulk lattice, grain boundaries and dislocation cores offer simple diffusion pathways. As a result, diffusion along defect centers requires less disruption of atomic bonds, which lowers activation energy routes. Nanocrystalline materials have a high density of short circuit diffusion routes due to their multiple surfaces. Thus, compared to single crystals or traditional polycrystals with the same chemical composition, nanocrystalline materials are anticipated to display higher self-diffusivity.
Enhanced solid solubility
The chemical potential, A, of a solute A in solvent B regulates the solubility of A in B. When compared to single crystals or glasses with the same chemical composition, nanocrystalline materials may have an improved (or reduced) chemical potential, which in turn affects how soluble A is in B.
Magnetic Properties
In recent years, there has been a lot of research done on magnetic nanoparticles. Their potential for use in important fields such as ultrahigh density magnetic storage systems, magnetic random access memory (MRAM), ferrofluids, spintronics, magnetic semiconductors, nanogranular magnetic materials, etc. is the primary factor behind this rising attention.
With greater interest arises the discovery that the magnetic properties of nanoparticles are mostly determined by their size and shape. A magnet’s coercivity and saturation magnetization levels determine how powerful it is. They increase when the specific surface area of the grains per unit volume increases and the grain size decreases.
Electrical Properties
The large grain boundary (surface) area of nanomaterials allows them to store far more energy than typical coarse-grained materials. The passage of an electric current through these materials or the application of an electric field can introduce a new optical absorption band or modify an existing band.
Many devices that require electrical power make use of both standard and rechargeable batteries. These batteries typically have a low energy density (storage capacity), necessitating frequent recharge. Because they are able to store far more energy than traditional ones, nanocrystalline materials are promising candidates for use as separator plates in batteries. It is expected that nickel- metal hydride batteries, which combine nanocrystalline nickel with metal hydrides, will last significantly longer and need charging a lot less frequently. Moreover, the dielectric properties are significantly enhanced by making these nanocrystalline.
In general words, nanomaterials are inferior to bulk materials in terms of thermal and electrical conductivity. According to the classical free electron theory of metals, electrical conductivity results from the free movement of electrons within a metallic solid. High electric-phonon and phonon-phonon scattering in nanomaterials is responsible for their low conductivity.
Optical Properties
The significantly distinct optical properties of nanocrystalline systems from those of bulk crystals have attracted a lot of attention. Nanoparticles’ ability to quantum-mechanically restrict electrical carriers, the efficiency with which energy and charge can be transferred over nanoscale distances, and the often greatly amplified role of interfaces are all important contributors. By manipulating the crystal size and surface chemistry, the linear and non-linear optical properties of such materials can be finely tuned.
The concepts of quantum confinement and surface plasmon processes provide an explanation for these unusual optical characteristics.
Quantum Confinement
The phenomenon known as quantum confinement is most apparent in semiconducting materials that have one dimension that falls within the range of 1-10 nanometers. Excitons are the electron-hole pairs that are present in a material, and the distance that separates them is referred to as the exciton-Bohr radius. In the case of bulk materials, the exciton-Bohr radius is significantly smaller than the size of the bulk material, whereas in the case of semiconducting materials, the exciton-Bohr radius is either smaller than or comparable to the size of the bulk material. Because exciton cannot extend to its natural limit, this results in it being constrained.
Quantum confinement is the term used to describe this occurrence. As a result of this process, the energy level of the valence band and the conduction band both become distinct, and the band gap energy shifts noticeably in comparison to the material in its bulk form. Band gap energy will decrease according to the size of the nanomaterial, and vice versa. This brings about a change in the optical properties known as a blue shift.
Surface Plasmon effect
Plasmons are quasiparticles that are formed when plasma oscillations are quantized in the same way that quantization of light and sound waves results in the formation of photons. When a photon interacts with plasmons, a new quasiparticle that is known as a plasma polariton can be created. Surface plasmons, often known as SP for short, are a subclass of plasmons that are confined to surfaces and form polariton through an intensive interaction with light. SPs are the elements to blame for the color of the nanomaterial.
The surface plasmon effect occurs when the size of the metallic particle grows smaller than the wavelength of light and the natural oscillation frequency of the plasmon becomes equal to the frequency of the light that is being used. This causes the surface plasmon to become excited or attain resonance, depending on which term you prefer.
As one illustration, the frequency of spherical gold particles is approximately 0.58 times higher than that of bulk plasma. Because of this, the frequency of the SP is in the visible range, despite the fact that the frequency of the bulk plasma is in the ultraviolet zone (with a wavelength close to 520 nm). The local electric field may be considerably increased close to an SP resonance if a wave of light is applied to a suspension of nanoparticles in a host. This can cause the SP resonance to occur.
Thermal Properties
In general, increasing the number of grain boundaries will result in increased phonon scattering at the disordered boundaries, which will lead to a reduction in the material’s thermal conductivity. Because of this, it is reasonable to anticipate that nanocrystalline materials will have a reduced heat conductivity compared to conventional materials. On the other hand, as the grain sizes approach nano dimensions, their dimensions start to resemble the mean free pathways of phonons, which are responsible for the transfer of thermal energy. As a result of the photon confinement and quantization effects of photon transport, nanomaterials can exhibit vastly different properties compared to coarse-grained materials.
These differences can be attributed to the size of the grains. It has been seen that the grain shape, in addition to the grain size, has an influence on the thermal characteristics of nanomaterials. This is something that has been discovered.
One-dimensional nanowires, for instance, may have extremely low thermal conductivities, which are quite different from the conductivities offered by carbon nanotubes. When compared to what is seen in bulk solids, the quantum confinement of phonons in one dimension can result in additional polarization modes when they are present in nanowires. The considerable drop in heat conductivity that nanostructures exhibit is due to the intense phonon–phonon interactions that occur as well as the amplified scattering that occurs at grain boundaries.
Many collective modes of phonon transport, in addition to the phonon modes in each individual layer, can develop in multilayered coatings, and the transport properties are greatly altered when the phonon coherence length becomes comparable to the thickness of each individual layer. Another intriguing application of nanomaterials’ thermal capabilities is the use of a nanofluid to improve thermal transport.
In simple words, nanostructured materials have a greater thermal expansion coefficient, which quantifies how the size of the material changes with temperature, than bulk materials do because of the high density of defects.
Mechanical Properties
Due to the essentially unique properties that nanocrystalline materials exhibit, there has been a lot of research done on the mechanical behavior of these materials. Because of the complex interaction of the defect structures, the tensile, work hardening, creep, fatigue, and deformation behaviors of nanocrystalline materials are very different from those of their bulk counterparts. This is owing to the fact that nanocrystalline materials are much smaller. The following is a list of the main factors that contribute to the significant differences in the mechanical behavior of nanostructured materials:
- The behavior of these defects in response to the application of stress is strongly impacted by the presence of a very high fraction of the atoms that reside at grain boundaries and triple junctions. The effect that grain size has on the stability of dislocations also has a substantial impact on the dynamics of dislocations, which in turn has repercussions for both the design of materials and their mechanical properties. Because dislocation interactions are the major deformation processes that determine the mechanical property of coarse-grained materials, the plastic deformation mode of nanomaterials may be drastically different. This is because dislocation interactions are responsible for determining the mechanical property of coarse-grained materials.
- Alternate deformation mechanisms such as grain boundary migration or sliding, crack expansion, and other similar processes become relevant for the reasons mentioned above.
- The amount of porosity in nanomaterials is significantly dependent on the processing technique that was used, and this porosity level can also be substantial, for instance in the case of specimens that were mechanically alloyed. Even after agglomeration, the pore size may still be less than the grain size or even the same. It’s possible that the presence of porosity will have a substantial impact on the way nanomaterials behave mechanically.
- Also, the mechanical characteristics of nanomaterials may be affected by the segregation of various solutes at the grain boundaries.
The greater density of defects such as grain boundaries, dislocations, triple junctions, etc., is what gives nanomaterials their distinctive mechanical properties due to the increased number of surface atoms and interfaces. When compared to bulk materials, nanomaterials have the following mechanical properties:
- Increased strength
- Increased toughness
- Increased hardness
- Increased ductility
Corrosion Properties
According to the findings of a number of studies, the drastically altered properties of nanocrystalline alloys are caused by the extremely high density of grain boundaries in these materials. Investigations into the corrosion of nanocrystalline materials revealed a more intense active anodic dissolution as compared to evaluations into corrosion involving conventional materials.
This distinct behavior can be attributed to the material’s small grain size, which creates a greater proportion of high-energy grain boundary defects than larger grain sizes do. This type of high-energy grain boundary site acts as a favored anodic dissolution site, which might consequently result in enhanced corrosion rates of nanocrystalline alloys. However, increased defect densities are not necessarily undesirable in every situation. Grain boundaries, which are easy diffusion pathways, can promote higher surface diffusion coefficients. This, in turn, can lead to the early production of a well-adherent and dense oxide layer, which in turn passivates the surface.
Just how small are nanomaterials? And what can we do with stuff that small? Watch out the video to learn special nanomaterial properties, how some can change at different sizes, and the difference between engineered nanomaterials and ones that occur naturally.
References
- Brechignac C., Houdy P., Lahmani M. (Eds.) Nanomaterials and Nanochemistry (Springer, 2007). ISBN 978-3-540-72993-8
- Nanochemistry, Biotechnology, Nanomaterials, and Their Applications. https://link.springer.com/book/10.1007/978-3-319-92567-7
- Al-Douri, Y. (2022). Nanomaterials Properties. In: Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-19-3881-8_1
- Cademartiri, Ludovico; Ozin, Geoffrey (2009). Concepts of Nanochemistry. Germany: Wiley VCH. pp. 4–7. ISBN 978-3527325979.
- Mochalin, Vadym N.; Shenderova, Olga; Ho, Dean; Gogotsi, Yury (2012-01-01). “The properties and applications of nanodiamonds”. Nature Nanotechnology.
- https://chemistnotes.com/nanochemistry/8-unique-properties-of-nanomaterials/
- Buzea, Cristina; Pacheco, Ivan; Robbie, Kevin (2007). “Nanomaterials and Nanoparticles: Sources and Toxicity”. Biointerphases.


thank you for your article